Iron Cycle on:
[Wikipedia]
[Google]
[Amazon]
 The iron cycle (Fe) is the
The iron cycle (Fe) is the


 The iron cycle interacts significantly with the sulfur, nitrogen, and phosphorus cycles. Soluble Fe(II) can act as the electron donor, reducing oxidized organic and inorganic electron receptors, including O2 and NO3, and become oxidized to Fe(III). The oxidized form of iron can then be the electron acceptor for reduced sulfur, H2, and organic carbon compounds. This returns the iron to the reduced Fe(II) state, completing the cycle.
The transition of iron between Fe(II) and Fe(III) in aquatic systems interacts with the freshwater
The iron cycle interacts significantly with the sulfur, nitrogen, and phosphorus cycles. Soluble Fe(II) can act as the electron donor, reducing oxidized organic and inorganic electron receptors, including O2 and NO3, and become oxidized to Fe(III). The oxidized form of iron can then be the electron acceptor for reduced sulfur, H2, and organic carbon compounds. This returns the iron to the reduced Fe(II) state, completing the cycle.
The transition of iron between Fe(II) and Fe(III) in aquatic systems interacts with the freshwater
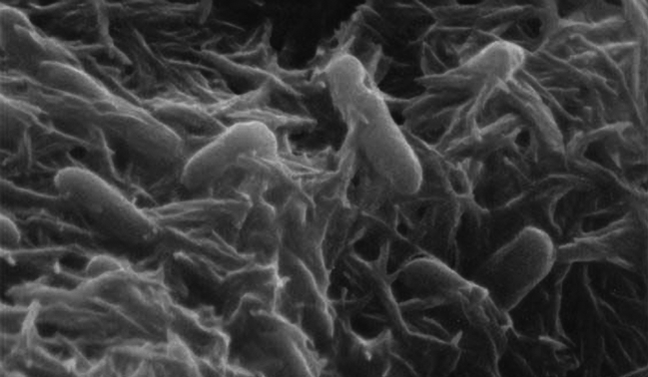 Another way bacteria influence the iron cycle is through extracellular electron transfer. Extracellular electron transfer occurs when a bacterium, in the absence of oxygen, utilizes metals in its environment as a final electron acceptor. This involves pumping electrons outside the cell to extracellular metals as a way to complete the
Another way bacteria influence the iron cycle is through extracellular electron transfer. Extracellular electron transfer occurs when a bacterium, in the absence of oxygen, utilizes metals in its environment as a final electron acceptor. This involves pumping electrons outside the cell to extracellular metals as a way to complete the
 te have augmented dust fluxes which increases the amount of aeolian dust to open regions of the ocean.Leeuwen, H. P. (Herman) van, Riemsdijk, W. H. van, Hiemstra, T. J. (Tjisse), Krebs, C. J., Hiemstra, T. J. (Tjisse), & Krebs, C. J. (2008). The biogeochemical cycle of Iron: The role of Natural Organic Matter. Other anthropogenic sources of iron are due to combustion. Highest combustion rates of iron occurs in East Asia, which contributes to 20-100% of ocean depositions around the globe.
Humans have altered the cycle for Nitrogen from fossil fuel combustion and large-scale agriculture. Due to increased Iron and Nitrogen raises marine nitrogen fixation in the subtropical North and South Pacific Ocean. In the subtropics, tropics and HNLC regions, increased inputs of iron may lead to increased CO2 uptake, impacting the global carbon cycle.
te have augmented dust fluxes which increases the amount of aeolian dust to open regions of the ocean.Leeuwen, H. P. (Herman) van, Riemsdijk, W. H. van, Hiemstra, T. J. (Tjisse), Krebs, C. J., Hiemstra, T. J. (Tjisse), & Krebs, C. J. (2008). The biogeochemical cycle of Iron: The role of Natural Organic Matter. Other anthropogenic sources of iron are due to combustion. Highest combustion rates of iron occurs in East Asia, which contributes to 20-100% of ocean depositions around the globe.
Humans have altered the cycle for Nitrogen from fossil fuel combustion and large-scale agriculture. Due to increased Iron and Nitrogen raises marine nitrogen fixation in the subtropical North and South Pacific Ocean. In the subtropics, tropics and HNLC regions, increased inputs of iron may lead to increased CO2 uptake, impacting the global carbon cycle.
 The iron cycle (Fe) is the
The iron cycle (Fe) is the biogeochemical cycle
A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the carbon cyc ...
of iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
through the atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
, hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to ch ...
, biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
and lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient
Micronutrients are essential chemicals required by organisms in small quantities to perform various biogeochemical processes and regulate physiological functions of cells and organs. By enabling these processes, micronutrients support the heal ...
in primary productivity
Primary or primaries may refer to:
Arts, entertainment, and media Music Groups and labels
* Primary (band), from Australia
* Primary (musician), hip hop musician and record producer from South Korea
* Primary Music, Israeli record label
Works
* ...
, and a limiting nutrient in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.
While iron can exist in a range of oxidation states
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms are fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Concep ...
from −2 to +7; however, on Earth it is predominantly in its +2 or +3 redox state. It is a primary redox-active metal in nature. The cycling of iron between its +2 and +3 oxidation states is referred to as the iron cycle. This process can be entirely abiotic
In biology and ecology, abiotic components or abiotic factors are non-living chemical and physical parts of the environment that affect living organisms and the functioning of ecosystems. Abiotic factors and the phenomena associated with them und ...
or facilitated by microorganisms
A microorganism, or microbe, is an organism of microscopic size, which may exist in its single-celled form or as a colony of cells. The possible existence of unseen microbial life was suspected from antiquity, with an early attestation in ...
, especially iron-oxidizing bacteria
Iron-oxidizing bacteria in surface water
Iron-oxidizing bacteria (or iron bacteria) are chemotrophic bacteria that derive energy by oxidizing dissolved iron. They are known to grow and proliferate in waters containing iron concentrations as low a ...
. The abiotic processes include the rust
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH) ...
ing of metallic which, in addition to oxidation of the metal, involves oxidation of Fe(II) in the presence of oxygen. Another type of abiotic process is the reduction of Fe3+ to Fe2+ by sulfide minerals. The biological cycling of Fe2+ is mediated by iron oxidizing and reducing microbes.
Iron is an essential micronutrient for life form. It is a key component of hemoglobin, important to nitrogen fixation as part of the Nitrogenase
Nitrogenases are enzymes () that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only fa ...
enzyme family, and as part of the iron-sulfur core of ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied t ...
it facilitates electron transport in chloroplasts, eukaryotic mitochondria, and bacteria. Due to the high reactivity of Fe2+ with oxygen and low solubility of Fe3+, iron is a limiting nutrient in most regions of the world.
Ancient earth
On the early Earth, when atmospheric oxygen levels were 0.001% of those present today, dissolved Fe2+ was thought to have been a lot more abundant in the oceans, and thus more bioavailable. Iron sulfide may have provided the energy and surfaces for the first organisms. Before the onset of oxygenicphotosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
, photo-ferrotrophs could obtain energy from sunlight, and use the electrons from Fe2+ to fix carbon dioxide.
During the Great Oxidation Event
The Great Oxidation Event (GOE) or Great Oxygenation Event, also called the Oxygen Catastrophe, Oxygen Revolution, Oxygen Crisis or Oxygen Holocaust, was a time interval during the Earth's Paleoproterozoic era when the Earth's atmosphere an ...
, 2.3-2.5 billion years ago, dissolved iron was oxidized by oxygen produced by cyanobacteria to form iron oxides. The iron oxides were denser than water and fell to the ocean floor forming banded iron formations (BIF). Over time, rising oxygen levels removed increasing amounts of iron from the ocean. BIFs have been a key source of iron ore in modern times.
Terrestrial ecosystems
The iron cycle is an important component of the terrestrial ecosystems. The ferrous form of iron, Fe2+, is dominant in the Earth's mantle, core, or deep crust. The ferric form, Fe3+, is more stable in the presence of oxygen gas. Dust is a key component in the Earth's iron cycle. Chemical and biologicalweathering
Weathering is the deterioration of rocks, soils and minerals (as well as wood and artificial materials) through contact with water, atmospheric gases, sunlight, and biological organisms. It occurs '' in situ'' (on-site, with little or no move ...
break down iron-bearing minerals, releasing the nutrient into the atmosphere. Changes in hydrological cycle and vegetative cover impact these patterns and have a large impact on global dust production, with dust deposition estimates ranging between 1000 and 2000 Tg/year. Aeolian dust
Dust is made of fine particles of solid matter. On Earth, it generally consists of particles in the atmosphere that come from various sources such as soil lifted by wind (an aeolian process), volcanic eruptions, and pollution.
Dust in home ...
is a critical part of the iron cycle by transporting iron particulates from the Earth's land via the atmosphere to the ocean.
Volcanic eruptions
A volcanic eruption occurs when material is expelled from a volcanic vent or fissure. Several types of volcanic eruptions have been distinguished by volcanologists. These are often named after famous volcanoes where that type of behavior h ...
also contribute to the terrestrial iron cycle, releasing iron-rich dust into the atmosphere in either a large burst or in smaller spurts over time. However, the distribution of volcanic ash is uneven across oceans and depends on the subduction zone. At higher temperatures within a volcano’s eruption plume, the interplay between volcanic ash particles and magmatic gases can determine the solubility of iron.The atmospheric transport of iron-rich dust can impact the ocean concentrations, enhancing the marine ecosystems productivity by fueling phytoplankton growth, increasing carbon uptake as well as the ocean-atmosphere CO2 exchange.
Oceanic ecosystem
The ocean is a critical component of the Earth'sclimate system
Earth's climate system is a complex system with five interacting components: the Atmosphere of Earth, atmosphere (air), the hydrosphere (water), the cryosphere (ice and permafrost), the lithosphere (earth's upper rocky layer) and the biosphere ( ...
, and the iron cycle plays a key role in ocean primary productivity and marine ecosystem function. Iron limitation has been known to limit the efficiency of the biological carbon pump. Iron limitation is a major factor controlling phytoplankton growth, particularly in High-Nutrient, Low-Chlorophyll (HNLC) regions, where iron availability restricts biological productivity despite abundant macronutrients. Increased anthropogenic iron deposition has altered marine primary production, particularly by stimulating nitrogen fixation in subtropical regions and influencing phytoplankton community structure. The largest supply of iron to the oceans is from rivers, where it is suspended as sediment particles. Coastal waters receive inputs of iron from rivers and anoxic sediments. Other major sources of iron to the ocean include glacial particulates, atmospheric dust transport, and hydrothermal vents
Hydrothermal vents are fissures on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hots ...
. Iron supply is an important factor affecting growth of phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater Aquatic ecosystem, ecosystems. The name comes from the Greek language, Greek words (), meaning 'plant', and (), mea ...
, the base of marine food web. Offshore regions rely on atmospheric dust deposition and upwelling. Other major sources of iron to the ocean include glacial particulates, hydrothermal vents, and volcanic ash. In offshore regions, bacteria also compete with phytoplankton for uptake of iron. In HNLC regions, iron limits the productivity of phytoplankton.
Most commonly, iron was available as an inorganic source to phytoplankton; however, organic forms of iron can also be used by specific diatoms
A diatom (Neo-Latin ''diatoma'') is any member of a large group comprising several Genus, genera of algae, specifically microalgae, found in the oceans, waterways and soils of the world. Living diatoms make up a significant portion of Earth's B ...
which use a process of surface reductase mechanism.
Role of marine animals in Iron cycling
ditIron uptake by phytoplankton leads to extremely low surface seawater iron concentrations, as iron is rapidly utilized in biological processes. Inorganic iron is the most common form available to phytoplankton, but specific diatoms can also access organic iron through surface reductase mechanisms.Remineralization
In biogeochemistry, remineralisation (or remineralization) refers to the breakdown or transformation of organic matter (those molecules derived from a biological source) into its simplest inorganic forms. These transformations form a crucial link ...
occurs when the sinking phytoplankton are degraded by zooplankton and bacteria. Upwelling recycles iron and causes higher deep water iron concentrations. On average there is 0.07±0.04 nmol Fe kg−1 at the surface (<200 m) and 0.76±0.25 nmol Fe kg−1 at depth (>500 m). Therefore, upwelling
Upwelling is an physical oceanography, oceanographic phenomenon that involves wind-driven motion of dense, cooler, and usually nutrient-rich water from deep water towards the ocean surface. It replaces the warmer and usually nutrient-depleted sur ...
zones contain more iron than other areas of the surface oceans. Soluble iron in ferrous form is bioavailable for utilization which commonly comes from aeolian resources.
Iron primarily exists in particulate phases as ferric iron, and the dissolved fraction is rapidly removed from the water column by coagulation. Consequently, the dissolved iron pool has a turnover time of approximately 100 years. The availability of soluble iron from aeolian sources is particularly important for sustaining biological activity in iron-limited regions. As anthropogenic activities continue to modify iron deposition patterns, the balance of marine biogeochemical cycles may shift, with potential consequences for global carbon sequestration and marine ecosystem dynamics.
Interactions with other elemental cycles
 The iron cycle interacts significantly with the sulfur, nitrogen, and phosphorus cycles. Soluble Fe(II) can act as the electron donor, reducing oxidized organic and inorganic electron receptors, including O2 and NO3, and become oxidized to Fe(III). The oxidized form of iron can then be the electron acceptor for reduced sulfur, H2, and organic carbon compounds. This returns the iron to the reduced Fe(II) state, completing the cycle.
The transition of iron between Fe(II) and Fe(III) in aquatic systems interacts with the freshwater
The iron cycle interacts significantly with the sulfur, nitrogen, and phosphorus cycles. Soluble Fe(II) can act as the electron donor, reducing oxidized organic and inorganic electron receptors, including O2 and NO3, and become oxidized to Fe(III). The oxidized form of iron can then be the electron acceptor for reduced sulfur, H2, and organic carbon compounds. This returns the iron to the reduced Fe(II) state, completing the cycle.
The transition of iron between Fe(II) and Fe(III) in aquatic systems interacts with the freshwater phosphorus cycle
The phosphorus cycle is the biogeochemical cycle that involves the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the moveme ...
. With oxygen in the water, Fe(II) gets oxidized to Fe(III), either abiotically or by microbes via lithotrophic oxidation. Fe(III) can form iron hydroxides, which bind tightly to phosphorus, removing it from the bioavailable phosphorus pool, limiting primary productivity. In anoxic conditions, Fe(III) can be reduced, used by microbes to be the final electron acceptor from either organic carbon or H2. This releases the phosphorus back into the water for biological use.
The iron and sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a consti ...
can interact at several points. Purple sulfur bacteria
The purple sulfur bacteria (PSB) are part of a group of Pseudomonadota capable of photosynthesis, collectively referred to as purple bacteria. They are anaerobic or microaerophilic, and are often found in stratified water environments includi ...
and green sulfur bacteria can use Fe(II) as an electron donor during anoxic photosynthesis. Sulfate reducing bacteria in anoxic environments can reduce sulfate to sulfide, which then binds to Fe(II) to create iron sulfide, a solid mineral that precipitates out of water and removes the iron and sulfur. The iron, phosphate, and sulfur cycles can all interact with each other. Sulfide can reduce Fe(III) from iron that is already bound to phosphate when there are no more metal ions available, which releases the phosphate and creates iron sulfide.
Iron plays an important role in the nitrogen cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, atmospheric, terrestrial ecosystem, terrestrial, and marine ecosystems. The conversion of nitrogen can ...
, aside from its role as part of the enzymes involved in nitrogen fixation. In anoxic conditions, Fe(II) can donate an electron that is accepted by NO3− which is oxidized to several different forms of nitrogen compounds, NO2−, N2O, N2, and NH4+, while Fe(II) is reduced to Fe(III).
Bacterial influences
Bacteria influence the iron cycle by performing redox reactions on free iron in their environment. These reactions can take place either inside the cell byiron-oxidizing bacteria
Iron-oxidizing bacteria in surface water
Iron-oxidizing bacteria (or iron bacteria) are chemotrophic bacteria that derive energy by oxidizing dissolved iron. They are known to grow and proliferate in waters containing iron concentrations as low a ...
or outside the cell through extracellular electron transfer.
Iron-oxidizing bacteria play an important role in the iron cycle by converting soluble ferrous iron
In chemistry, iron(II) refers to the element iron in its +2 oxidation state. The adjective ''ferrous'' or the prefix ''ferro-'' is often used to specify such compounds, as in ''ferrous chloride'' for iron(II) chloride (). The adjective ''ferr ...
, Fe (II), into insoluble ferric iron, Fe (III). Iron-oxidizing bacteria can be both autotrophic
An autotroph is an organism that can convert abiotic sources of energy into energy stored in organic compounds, which can be used by other organisms. Autotrophs produce complex organic compounds (such as carbohydrates, fats, and proteins) us ...
and heterotrophic
A heterotroph (; ) is an organism that cannot produce its own food, instead taking nutrition from other sources of organic carbon, mainly plant or animal matter. In the food chain, heterotrophs are primary, secondary and tertiary consumers, but ...
and are most prevalent in de-oxygenated environments because the lack of oxygen favors organisms with a different final electron acceptor. Examples of iron-oxidizing bacteria include '' Acidithiobacillus ferrooxidans'', ''Gallionella ferruginea'', and ''Rhodopseudomonas
''Rhodopseudomonas'' is a genus of bacteria from the family Nitrobacteraceae.
Phylogeny
The currently accepted taxonomy is based on the List of Prokaryotic names with Standing in Nomenclature
List of Prokaryotic names with Standing in Nomen ...
'' spp. ''.'' The consequences of iron-oxidizing bacteria are that there is a smaller concentration of soluble iron in the environment. This leads to less plant growth, discoloration of streams, and decreased sequestration of free phosphorus ions.
 Another way bacteria influence the iron cycle is through extracellular electron transfer. Extracellular electron transfer occurs when a bacterium, in the absence of oxygen, utilizes metals in its environment as a final electron acceptor. This involves pumping electrons outside the cell to extracellular metals as a way to complete the
Another way bacteria influence the iron cycle is through extracellular electron transfer. Extracellular electron transfer occurs when a bacterium, in the absence of oxygen, utilizes metals in its environment as a final electron acceptor. This involves pumping electrons outside the cell to extracellular metals as a way to complete the electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
. One popular metal to perform extracellular electron transport to is Fe (III). Examples of bacteria such as ''Shewanella'' spp., ''Geobacter'' spp., and some ''Enterococcus'' spp. use this form of metabolism. This is an important metabolic process because it reduces ferric iron to ferrous iron, which is more readily bioavailable. Thus, extracellular electron transport has similar effects on the iron cycle as iron-oxidizing bacteria. Besides the iron cycle, extracellular electron transport also impacts the environment through increased biofilm
A biofilm is a Syntrophy, syntrophic Microbial consortium, community of microorganisms in which cell (biology), cells cell adhesion, stick to each other and often also to a surface. These adherent cells become embedded within a slimy ext ...
growth and increased biofilm acidity. Moving away from the Earth, it has been theorized that bacteria that can undergo extracellular electron transfer may exist on Mars
Mars is the fourth planet from the Sun. It is also known as the "Red Planet", because of its orange-red appearance. Mars is a desert-like rocky planet with a tenuous carbon dioxide () atmosphere. At the average surface level the atmosph ...
due to Mars’ high iron oxide mineral content.
Taken together these two metabolic processes underscore the important role bacteria have in the iron cycle and the environment as a whole.
Anthropogenic influences
Human impact on the iron cycle in the ocean is due to dust concentrations increasing at the beginning of the industrial era. Today, there is approximately double the amount of soluble iron in oceans than pre-industrial times from anthropogenic pollutants and soluble iron combustion sources. Changes in human land-use activities and clima te have augmented dust fluxes which increases the amount of aeolian dust to open regions of the ocean.Leeuwen, H. P. (Herman) van, Riemsdijk, W. H. van, Hiemstra, T. J. (Tjisse), Krebs, C. J., Hiemstra, T. J. (Tjisse), & Krebs, C. J. (2008). The biogeochemical cycle of Iron: The role of Natural Organic Matter. Other anthropogenic sources of iron are due to combustion. Highest combustion rates of iron occurs in East Asia, which contributes to 20-100% of ocean depositions around the globe.
Humans have altered the cycle for Nitrogen from fossil fuel combustion and large-scale agriculture. Due to increased Iron and Nitrogen raises marine nitrogen fixation in the subtropical North and South Pacific Ocean. In the subtropics, tropics and HNLC regions, increased inputs of iron may lead to increased CO2 uptake, impacting the global carbon cycle.
te have augmented dust fluxes which increases the amount of aeolian dust to open regions of the ocean.Leeuwen, H. P. (Herman) van, Riemsdijk, W. H. van, Hiemstra, T. J. (Tjisse), Krebs, C. J., Hiemstra, T. J. (Tjisse), & Krebs, C. J. (2008). The biogeochemical cycle of Iron: The role of Natural Organic Matter. Other anthropogenic sources of iron are due to combustion. Highest combustion rates of iron occurs in East Asia, which contributes to 20-100% of ocean depositions around the globe.
Humans have altered the cycle for Nitrogen from fossil fuel combustion and large-scale agriculture. Due to increased Iron and Nitrogen raises marine nitrogen fixation in the subtropical North and South Pacific Ocean. In the subtropics, tropics and HNLC regions, increased inputs of iron may lead to increased CO2 uptake, impacting the global carbon cycle.
See also
* Iron fertilization *Iron-oxidizing bacteria
Iron-oxidizing bacteria in surface water
Iron-oxidizing bacteria (or iron bacteria) are chemotrophic bacteria that derive energy by oxidizing dissolved iron. They are known to grow and proliferate in waters containing iron concentrations as low a ...
References
Further reading
* {{Biogeochemical cycle Biogeochemical cycle Geological processes Iron