Intermediate Filaments on:
[Wikipedia]
[Google]
[Amazon]
Intermediate filaments (IFs) are
 The structure of proteins that form intermediate filaments (IF) was first predicted by computerized analysis of the
The structure of proteins that form intermediate filaments (IF) was first predicted by computerized analysis of the
 These proteins are the most diverse among IFs and constitute type I (acidic) and type II (basic) IF proteins. The many
These proteins are the most diverse among IFs and constitute type I (acidic) and type II (basic) IF proteins. The many
cytoskeletal
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all Cell (biology), cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane ...
structural components found in the cells of vertebrate
Vertebrates () are animals with a vertebral column (backbone or spine), and a cranium, or skull. The vertebral column surrounds and protects the spinal cord, while the cranium protects the brain.
The vertebrates make up the subphylum Vertebra ...
s, and many invertebrate
Invertebrates are animals that neither develop nor retain a vertebral column (commonly known as a ''spine'' or ''backbone''), which evolved from the notochord. It is a paraphyletic grouping including all animals excluding the chordata, chordate s ...
s. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate ''Branchiostoma
''Branchiostoma'' is one of the few living genera of lancelets ( order Amphioxiformes). It is the type genus of family Branchiostomatidae.
These small vaguely eel- or snake-like animals are close relatives of vertebrates. The scientific name m ...
''.
Intermediate filaments are composed of a family of related protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s sharing common structural and sequence features. Initially designated 'intermediate' because their average diameter (10 nm) is between those of narrower microfilament
Microfilaments, also called actin filaments, are protein filaments in the cytoplasm of eukaryotic cells that form part of the cytoskeleton. They are primarily composed of polymers of actin, but are modified by and interact with numerous other ...
s (actin) and wider myosin
Myosins () are a Protein family, family of motor proteins (though most often protein complexes) best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are adenosine triphosphate, ATP- ...
filaments found in muscle cells, the diameter of intermediate filaments is now commonly compared to actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of ...
microfilaments (7 nm) and microtubule
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nanometer, nm and have an inner diameter bet ...
s (25 nm). Animal intermediate filaments are subcategorized into six types based on similarities in amino acid sequence and protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
structure. Most types are cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
ic, but one type, Type V is a nuclear lamin. Unlike microtubules, IF distribution in cells shows no good correlation with the distribution of either mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is us ...
or endoplasmic reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryote, eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for ...
.
Structure
amino acid sequence
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthe ...
of a human epidermal keratin
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. It is the key structural material making up Scale (anatomy), scales, hair, Nail (anatomy), nails, feathers, horn (anatomy), horns, claws, Hoof, hoove ...
derived from cloned cDNAs. Analysis of a second keratin sequence revealed that the two types of keratins share only about 30% amino acid sequence homology but share similar patterns of secondary structure domains. As suggested by the first model, all IF proteins appear to have a central alpha-helical rod domain that is composed of four alpha-helical segments (named as 1A, 1B, 2A and 2B) separated by three linker regions.
The central building block of an intermediate filament is a pair of two intertwined proteins that is called a coiled-coil structure. This name reflects the fact that the structure of each protein is helical, and the intertwined pair is also a helical structure. Structural analysis of a pair of keratins shows that the two proteins that form the coiled-coil bind by hydrophobic interactions. The charged residues in the central domain do not have a major role in the binding of the pair in the central domain.
Cytoplasmic IFs assemble into non-polar unit-length filaments (ULFs). Identical ULFs associate laterally into staggered, antiparallel, soluble tetramers, which associate head-to-tail into protofilaments that pair up laterally into protofibrils, four of which wind together into an intermediate filament.
Part of the assembly process includes a compaction step, in which ULF tighten and assume a smaller diameter. The reasons for this compaction are not well understood, and IF are routinely observed to have diameters ranging between 6 and 12 nm.
The N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amin ...
and the C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein
Proteins are large biomolecules and macromolecules that comp ...
of IF proteins are non-alpha-helical regions and show wide variation in their lengths and sequences across IF families.
The N-terminal "head domain" binds DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
. Vimentin
Vimentin is a structural protein that in humans is encoded by the ''VIM'' gene. Its name comes from the Latin ''vimentum'' which refers to an array of flexible rods.
Vimentin is a Intermediate filament#Type III, type III intermediate filamen ...
heads are able to alter nuclear
Nuclear may refer to:
Physics
Relating to the nucleus of the atom:
*Nuclear engineering
*Nuclear physics
*Nuclear power
*Nuclear reactor
*Nuclear weapon
*Nuclear medicine
*Radiation therapy
*Nuclear warfare
Mathematics
* Nuclear space
*Nuclear ...
architecture and chromatin
Chromatin is a complex of DNA and protein found in eukaryote, eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important r ...
distribution, and the liberation of heads by HIV-1
The subtypes of HIV include two main subtypes, known as HIV type 1 (HIV-1) and HIV type 2 (HIV-2). These subtypes have distinct genetic differences and are associated with different epidemiological patterns and clinical characteristics.
HIV-1 e ...
protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalysis, catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products ...
may play an important role in HIV-1 associated cytopathogenesis and carcinogenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cell (biology), cells are malignant transformation, transformed into cancer cells. The process is characterized by changes at the cellular, G ...
. Phosphorylation
In biochemistry, phosphorylation is described as the "transfer of a phosphate group" from a donor to an acceptor. A common phosphorylating agent (phosphate donor) is ATP and a common family of acceptor are alcohols:
:
This equation can be writ ...
of the head region can affect filament stability. The head has been shown to interact with the rod domain of the same protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
.
C-terminal "tail domain" shows extreme length variation between different IF proteins.
The anti-parallel orientation of tetramers means that, unlike microtubules and microfilaments, which have a plus end and a minus end, IFs lack polarity and cannot serve as basis for cell motility and intracellular transport.
Also, unlike actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of ...
or tubulin
Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. ╬▒- and ╬▓-tubulins polymerize into microtubules, a major component of the eukaryotic cytosk ...
, intermediate filaments do not contain a binding site
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may includ ...
for a nucleoside triphosphate
A nucleoside triphosphate is a nucleoside containing a nitrogenous base bound to a 5-carbon sugar (either ribose or deoxyribose), with three phosphate groups bound to the sugar. They are the molecular precursors of both DNA and RNA, which are chai ...
.
Cytoplasmic IFs do not undergo treadmilling like microtubules and actin fibers, but are dynamic.
Biomechanical properties
IFs are rather deformable proteins that can be stretched several times their initial length. The key to facilitate this large deformation is due to their hierarchical structure, which facilitates a cascaded activation of deformation mechanisms at different levels of strain. Initially the coupled alpha-helices of unit-length filaments uncoil as they're strained, then as the strain increases they transition intobeta-sheet
The beta sheet (╬▓-sheet, also ╬▓-pleated sheet) is a common structural motif, motif of the regular protein secondary structure. Beta sheets consist of beta strands (╬▓-strands) connected laterally by at least two or three backbone chain, backbon ...
s, and finally at increased strain the hydrogen bonds between beta-sheets slip and the ULF monomers slide along each other.
Types
There are about 70 different human genes coding for various intermediate filament proteins. However, different kinds of IFs share basic characteristics: In general, they are all polymers that measure between 9ŌĆō11 nm in diameter when fully assembled. Animal IFs are subcategorized into six types based on similarities in amino acid sequence andprotein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
structure:
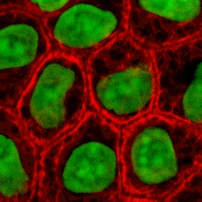
Types I and II ŌĆō acidic and basic keratins
 These proteins are the most diverse among IFs and constitute type I (acidic) and type II (basic) IF proteins. The many
These proteins are the most diverse among IFs and constitute type I (acidic) and type II (basic) IF proteins. The many isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have uniqu ...
s are divided in two groups:
* epithelial keratins (about 20) in epithelial
Epithelium or epithelial tissue is a thin, continuous, protective layer of cells with little extracellular matrix. An example is the epidermis, the outermost layer of the skin. Epithelial ( mesothelial) tissues line the outer surfaces of man ...
cells (image to right)
* trichocytic keratins (about 13) ( hair keratins), which make up hair
Hair is a protein filament that grows from follicles found in the dermis. Hair is one of the defining characteristics of mammals.
The human body, apart from areas of glabrous skin, is covered in follicles which produce thick terminal and ...
, nails, horns and reptilian scales
Scale or scales may refer to:
Mathematics
* Scale (descriptive set theory), an object defined on a set of points
* Scale (ratio), the ratio of a linear dimension of a model to the corresponding dimension of the original
* Scale factor, a number ...
.
Regardless of the group, keratins are either acidic or basic. Acidic and basic keratins bind each other to form acidic-basic heterodimers and these heterodimers then associate to make a keratin filament.
Cytokeratin
Cytokeratins are keratin proteins found in the intracytoplasmic cytoskeleton of epithelial tissue. They are an important component of intermediate filaments, which help cells resist mechanical stress. Expression of these cytokeratins within ep ...
filaments laterally associate with each other to create a thick bundle of ~50 nm radius. The optimal radius of such bundles is determined by the interplay between the long range electrostatic repulsion and short range hydrophobic attraction. Subsequently, these bundles would intersect through junctions to form a dynamic network, spanning the cytoplasm of epithelial cells.
Type III
There are four proteins classed as type III intermediate filament proteins, which may form homo- or heteropolymeric proteins. *Desmin
Desmin is a protein that in humans is encoded by the ''DES'' gene. Desmin is a muscle-specific, type III intermediate filament that integrates the sarcolemma, Z disk, and nuclear membrane in sarcomeres and regulates sarcomere architecture.
...
IFs are structural components of the sarcomere
A sarcomere (Greek Žā╬¼Žü╬Š ''sarx'' "flesh", ╬╝╬ŁŽü╬┐Žé ''meros'' "part") is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal striated muscle, Skeletal muscles are composed of tubular ...
s in muscle cells and connect different cell organelles like the desmosomes with the cytoskeleton.
* Glial fibrillary acidic protein
Glial fibrillary acidic protein (GFAP) is a protein that is encoded by the ''GFAP'' gene in humans. It is a type III intermediate filament (IF) protein that is expressed by numerous cell types of the central nervous system (CNS), including astro ...
(GFAP) is found in astrocyte
Astrocytes (from Ancient Greek , , "star" and , , "cavity", "cell"), also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of en ...
s and other glia
Glia, also called glial cells (gliocytes) or neuroglia, are non-neuronal cells in the central nervous system (the brain and the spinal cord) and in the peripheral nervous system that do not produce electrical impulses. The neuroglia make up ...
.
* Peripherin found in peripheral neurons.
* Vimentin
Vimentin is a structural protein that in humans is encoded by the ''VIM'' gene. Its name comes from the Latin ''vimentum'' which refers to an array of flexible rods.
Vimentin is a Intermediate filament#Type III, type III intermediate filamen ...
, the most widely distributed of all IF proteins, can be found in fibroblast
A fibroblast is a type of cell (biology), biological cell typically with a spindle shape that synthesizes the extracellular matrix and collagen, produces the structural framework (Stroma (tissue), stroma) for animal Tissue (biology), tissues, and ...
s, leukocytes
White blood cells (scientific name leukocytes), also called immune cells or immunocytes, are cells of the immune system that are involved in protecting the body against both infectious disease and foreign entities. White blood cells are genera ...
, and blood vessel endothelial cell
The endothelium (: endothelia) is a single layer of squamous endothelial cells that line the interior surface of blood vessels and lymphatic vessels. The endothelium forms an interface between circulating blood or lymph in the lumen and th ...
s. They support the cellular membranes, keep some organelle
In cell biology, an organelle is a specialized subunit, usually within a cell (biology), cell, that has a specific function. The name ''organelle'' comes from the idea that these structures are parts of cells, as Organ (anatomy), organs are to th ...
s in a fixed place within the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
, and transmit membrane receptor signals to the nucleus.
* Syncoilin
Discovery
Syncoilin is a muscle-specific atypical Intermediate filament#Type III, type III intermediate filament protein encoded in the human by the gene ''SYNC''. It was first isolated as a binding partner to DTNA, ╬▒-dystrobrevin, as determine ...
is an atypical type III IF protein.
Type IV
* Alpha-internexin *Neurofilament
Neurofilaments (NF) are classed as Intermediate filament#Type IV, type IV intermediate filaments found in the cytoplasm of neurons. They are protein polymers measuring 10 nm in diameter and many micrometers in length. Together with mic ...
s ŌĆō the type IV family of intermediate filaments that is found in high concentrations along the axon
An axon (from Greek ß╝ä╬ŠŽē╬Į ''├Īx┼Źn'', axis) or nerve fiber (or nerve fibre: see American and British English spelling differences#-re, -er, spelling differences) is a long, slender cellular extensions, projection of a nerve cell, or neuron, ...
s of vertebrate neurons.
* Synemin
* Syncoilin
Discovery
Syncoilin is a muscle-specific atypical Intermediate filament#Type III, type III intermediate filament protein encoded in the human by the gene ''SYNC''. It was first isolated as a binding partner to DTNA, ╬▒-dystrobrevin, as determine ...
Type V ŌĆō nuclear lamins
*Lamin
Lamins, also known as nuclear lamins, are fibrous proteins in Intermediate filament#Type V ŌĆō nuclear lamins, type V intermediate filaments, providing structural function and Transcription (biology), transcriptional regulation in the cell nucle ...
s
Lamins are fibrous proteins having structural function in the cell nucleus.
In metazoan cells, there are A and B type lamins, which differ in their length and pI. Human cells have three differentially regulated genes.
B-type lamins are present in every cell. B type lamins, lamin B1
Lamin-B1 is a protein that in humans is encoded by the ''LMNB1'' gene.
The nuclear lamina consists of a two-dimensional matrix of proteins located next to the inner nuclear membrane. The lamin family of proteins make up the matrix and are highly ...
and B2, are expressed from the LMNB1 and LMNB2 genes on 5q23 and 19q13, respectively.
A-type lamins are only expressed following gastrulation
Gastrulation is the stage in the early embryonic development of most animals, during which the blastula (a single-layered hollow sphere of cells), or in mammals, the blastocyst, is reorganized into a two-layered or three-layered embryo known as ...
. Lamin A and C are the most common A-type lamins and are splice variants of the LMNA gene found at 1q21.
These proteins localize to two regions of the nuclear compartment, the nuclear laminaŌĆöa proteinaceous structure layer subjacent to the inner surface of the nuclear envelope
The nuclear envelope, also known as the nuclear membrane, is made up of two lipid bilayer membranes that in eukaryotic cells surround the nucleus, which encloses the genetic material.
The nuclear envelope consists of two lipid bilayer membran ...
and throughout the nucleoplasm in the nucleoplasmic veil.
Comparison of the lamins to vertebrate cytoskeletal IFs shows that lamins have an extra 42 residues (six heptads) within coil 1b. The c-terminal tail domain contains a nuclear localization signal (NLS), an Ig-fold-like domain, and in most cases a carboxy-terminal CaaX box that is isoprenylated and carboxymethylated (lamin C does not have a CAAX box). Lamin A is further processed to remove the last 15 amino acids and its farnesylated cysteine.
During mitosis, lamins are phosphorylated by MPF, which drives the disassembly of the lamina and the nuclear envelope.
Type VI
* Beaded filaments: Filensin, Phakinin. * Nestin (was once proposed for reclassification but due to differences, remains as a type VI IF protein) Vertebrate-only. Related to type I-IV. Used to contain other newly discovered IF proteins not yet assigned to a type.Function
Cell adhesion
At theplasma membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
, some keratins or desmin interact with desmosome
A desmosome (; "binding body"), also known as a macula adherens (plural: maculae adherentes) (Latin for ''adhering spot''), is a cell structure specialized for cell-to-cell adhesion. A type of junctional complex, they are localized spot-like ad ...
s (cell-cell adhesion) and hemidesmosome
Hemidesmosomes are very small stud-like structures found in keratinocytes of the epidermis of skin that attach to the extracellular matrix. They are similar in form to desmosomes when visualized by electron microscopy; however, desmosomes attach ...
s (cell-matrix adhesion) via adapter proteins.
Associated proteins
Filaggrin binds to keratin fibers in epidermal cells.Plectin
Plectin is a giant protein found in nearly all mammalian cells which acts as a link between the three main components of the cytoskeleton: actin microfilaments, microtubules and intermediate filaments. In addition, plectin links the cytoskeleto ...
links vimentin to other vimentin fibers, as well as to microfilaments, microtubules, and myosin
Myosins () are a Protein family, family of motor proteins (though most often protein complexes) best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are adenosine triphosphate, ATP- ...
II. Kinesin is being researched and is suggested to connect vimentin to tubulin via motor proteins.
Keratin filaments in epithelial cells link to desmosome
A desmosome (; "binding body"), also known as a macula adherens (plural: maculae adherentes) (Latin for ''adhering spot''), is a cell structure specialized for cell-to-cell adhesion. A type of junctional complex, they are localized spot-like ad ...
s (desmosomes connect the cytoskeleton together) through plakoglobin
Plakoglobin, also known as junction plakoglobin or gamma-catenin, is a protein that in humans is encoded by the ''JUP'' gene. Plakoglobin is a member of the catenin protein family and homologous to ╬▓-catenin. Plakoglobin is a cytoplasmic compon ...
, desmoplakin
Desmoplakin is a protein in humans that is encoded by the ''DSP'' gene. Desmoplakin is a critical component of desmosome structures in cardiac muscle and epidermal cells, which function to maintain the structural integrity at adjacent cell co ...
, desmoglein
The desmogleins are a family of desmosomal cadherins consisting of proteins DSG1, DSG2, DSG3, and DSG4. They play a role in the formation of desmosomes that join cells to one another.
Pathology
Desmogleins are targeted in the autoimmune disease ...
s, and desmocollins; desmin
Desmin is a protein that in humans is encoded by the ''DES'' gene. Desmin is a muscle-specific, type III intermediate filament that integrates the sarcolemma, Z disk, and nuclear membrane in sarcomeres and regulates sarcomere architecture.
...
filaments are connected in a similar way in heart muscle cells.
Diseases arising from mutations in IF genes
* Dilated cardiomyoathy (DCM), mutations in the ''DES'' gene * Arrhythmogenic cardiomyopathy (ACM), mutations in the ''DES'' gene * Restrictive cardiomyopathy (RCM), mutations in the ''DES'' gene * Non-compaction cardiomyopathy, mutations in the ''DES'' genes * Cardiomyopathy in combination with skeletal myopathy (''DES'') *Epidermolysis bullosa simplex
Epidermolysis bullosa simplex (EBS) is a disorder resulting from mutations in the genes encoding keratin 5 or keratin 14.Freedberg, et al. (2003). ''Fitzpatrick's Dermatology in General Medicine''. (6th ed.). McGraw-Hill. . It is one of the major f ...
; keratin 5
Keratin 5, also known as KRT5, K5, or CK5, is a protein that is encoded in humans by the ''KRT5'' gene. It protein dimer, dimerizes with keratin 14 and forms the intermediate filaments (IF) that make up the cytoskeleton of Stratum basale, basal ...
or keratin 14 mutation
* Laminopathies
Laminopathies ('' lamino-'' + '' -pathy'') are a group of rare genetic disorders caused by mutations in genes encoding proteins of the nuclear lamina. Since the first reports of laminopathies in the late 1990s, increased research efforts have sta ...
are a family of diseases caused by mutations in nuclear lamins and include Hutchinson-Gilford progeria syndrome and various lipodystrophies and cardiomyopathies among others.
In other organisms
IF proteins are universal among animals in the form of a nuclear lamin. The Hydra has an additional "nematocilin" derived from the lamin. Cytoplasmic IFs (type I-IV) are only found inBilateria
Bilateria () is a large clade of animals characterised by bilateral symmetry during embryonic development. This means their body plans are laid around a longitudinal axis with a front (or "head") and a rear (or "tail") end, as well as a leftŌĆ ...
; they also arose from a gene duplication
Gene duplication (or chromosomal duplication or gene amplification) is a major mechanism through which new genetic material is generated during molecular evolution. It can be defined as any duplication of a region of DNA that contains a gene ...
event involving "type V" nuclear lamin. In addition, a few other diverse types of eukaryotes have lamins, suggesting an early origin of the protein.
There was not really a concrete definition of an "intermediate filament protein", in the sense that the size or shape-based definition does not cover a monophyletic group. With the inclusion of unusual proteins like the network-forming beaded lamins (type VI), the current classification is moving to a clade containing nuclear lamin and its many descendants, characterized by sequence similarity as well as the exon structure. Functionally-similar proteins out of this clade, like crescentins, alveolins, tetrins, and epiplasmins, are therefore only "IF-like". They likely arose through convergent evolution
Convergent evolution is the independent evolution of similar features in species of different periods or epochs in time. Convergent evolution creates analogous structures that have similar form or function but were not present in the last comm ...
.
References
Further reading
* * *External links
* {{DEFAULTSORT:Intermediate Filament Protein families Cytoskeleton