Imes on:
[Wikipedia]
[Google]
[Amazon]
IMes is an abbreviation for an
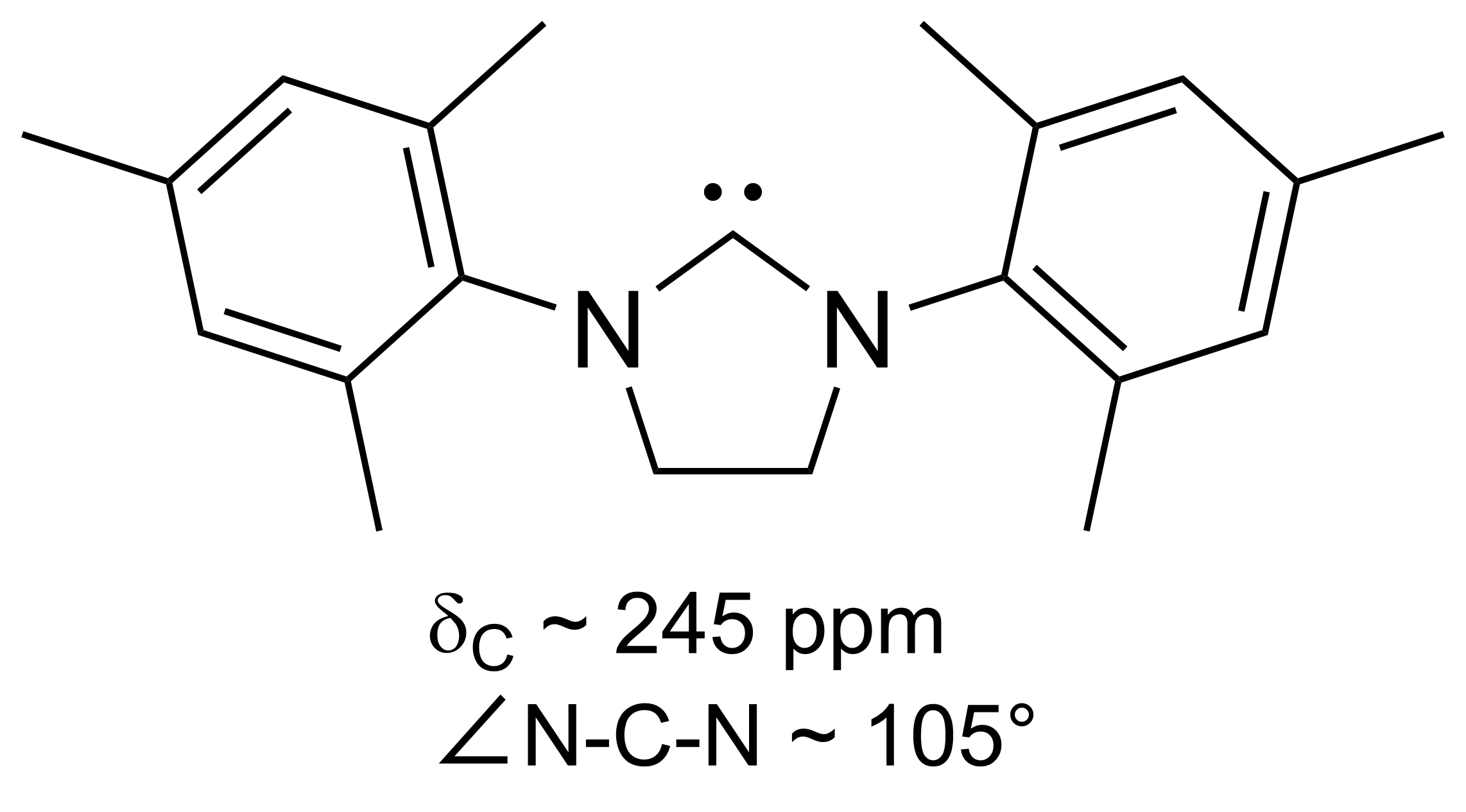
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
that is a common ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
in organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
. It is an ''N''-heterocyclic carbene (NHC). The compound, a white solid, is often not isolated but instead is generated upon attachment to the metal centre.
First prepared by Arduengo, the heterocycle is synthesized by condensation of 2,4,6-trimethylaniline
2,4,6-Trimethylaniline is an organic compound with formula (CH3)3C6H2NH2. It is an aromatic amine that is of commercial interest as a precursor to dyes. It is prepared by selective nitration of mesitylene, avoiding oxidation of the methyl groups, ...
and glyoxal
Glyoxal is an organic compound with the chemical formula OCHCHO. It is the smallest dialdehyde (a compound with two aldehyde groups). It is a crystalline solid, white at low temperatures and yellow near the melting point (15 °C). The liquid ...
to give the diimine
Diimines are organic compounds containing two imine (RCH=NR') groups. Common derivatives are 1,2-diimines and 1,3-diimines. These compounds are used as ligands, but they are also precursors to other organic compounds.
Preparation
Diimines are ...
. In the presence of acid, the resulting glyoxal-bis(mesitylimine)
Glyoxal-bis(mesitylimine) is an organic compound with the formula H2C2(NC6H2Me3)2 (Me = methyl). It is a yellow solid that is soluble in organic solvents. It is classified as a diimine ligand. It is used in coordination chemistry and homogeneous c ...
condenses with formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
to give the dimesityl imidazolium cation. This cation is the conjugate acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the rever ...
of the NHC.
Related compounds
Bulkier than IMes is the NHC ligand IPr ( CAS 244187-81-3). IPr features diisopropylphenyl in place of the mesityl substituents. Some variants of IMes and IPr have saturated backbones, two such ligands areSIMes
SIMes (or H2Imes) is an N-heterocyclic carbene, ''N''-heterocyclic carbene. It is a white solid that dissolves in organic solvents. The compound is used as a ligand in organometallic chemistry. It is structurally related to the more common ligan ...
and SIPr. They are prepared by alkylation of substituted anilines with dibromoethane followed by ring closure and dehydrohalogenation of the dihydroimidazolium salt.
:
References
Further reading
* {{Organometallics Organometallic chemistry Carbenes Ligands