Hydrated Lime on:
[Wikipedia]
[Google]
[Amazon]
Calcium hydroxide (traditionally called slaked lime) is an
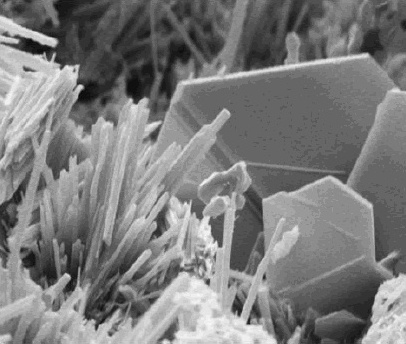 Calcium hydroxide adopts a polymeric structure, as do all metal hydroxides. The structure is identical to that of Mg(OH) (''brucite structure''); i.e., the cadmium iodide motif. Strong
Calcium hydroxide adopts a polymeric structure, as do all metal hydroxides. The structure is identical to that of Mg(OH) (''brucite structure''); i.e., the cadmium iodide motif. Strong
 In Nahuatl, the language of the Aztecs, the word for calcium hydroxide is ''nextli''. In a process called ''
In Nahuatl, the language of the Aztecs, the word for calcium hydroxide is ''nextli''. In a process called ''
CDC – NIOSH Pocket Guide to Chemical Hazards – Calcium Hydroxide
*
MSDS Data Sheet
{{DEFAULTSORT:Calcium Hydroxide Building materials Calcium compounds Dental materials Hydroxides Inorganic compounds Intoxication E-number additives
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemis ...
with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "'' lime''" connotes calcium-containing inorganic ...
) is mixed or slaked with water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number
E numbers ("E" stands for "Europe") are codes for substances used as food additives, including those found naturally in many foods such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly ...
E526. Limewater
Limewater is the common name for a saturated aqueous solution of calcium hydroxide. Calcium hydroxide, Ca(OH)2, is sparsely soluble at room temperature in water (1.5 g/L at 25 °C). "Pure" (i.e. less than or fully saturated) limewater i ...
, also called milk of lime, is the common name for a saturated solution
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
of calcium hydroxide.
Properties
Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. With a solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following dissolution reaction: : Ca(OH)2 → Ca2+ + 2 OH− At ambient temperature, calcium hydroxide (portlandite
Portlandite is a hydroxide-bearing mineral typically included in the oxide mineral class. It is the naturally occurring form of calcium hydroxide (Ca(OH)2) and the calcium analogue of brucite (Mg(OH)2).
Occurrence
Portlandite occurs in a variety ...
) dissolves in pure water to produce an alkaline solution with a pH of about 12.5. Calcium hydroxide solutions can cause chemical burns. At high pH values due to a common-ion effect with the hydroxide anion , its solubility drastically decreases. This behavior is relevant to cement pastes. Aqueous solutions of calcium hydroxide are called limewater
Limewater is the common name for a saturated aqueous solution of calcium hydroxide. Calcium hydroxide, Ca(OH)2, is sparsely soluble at room temperature in water (1.5 g/L at 25 °C). "Pure" (i.e. less than or fully saturated) limewater i ...
and are medium-strength bases, which react with acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a se ...
s and can attack some metal
A metal (from ancient Greek, Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, e ...
s such as aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
( amphoteric hydroxide dissolving at high pH), while protecting other metals, such as iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
and steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistan ...
, from corrosion by passivation of their surface. Limewater turns milky in the presence of carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
due to the formation of insoluble calcium carbonate, a process called carbonatation
Carbonatation is a chemical reaction in which calcium hydroxide reacts with carbon dioxide and forms insoluble calcium carbonate:
:Ca(OH)2CO2->CaCO3H_2O
The process of forming a carbonate is sometimes referred to as "carbonation", although t ...
:
: Ca(OH)2 + CO2 → CaCO3 + H2O
When heated to 512 °C, the partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
of water in equilibrium with calcium hydroxide reaches 101kPa (normal atmospheric pressure), which decomposes calcium hydroxide into calcium oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "'' lime''" connotes calcium-containing inorganic ...
and water:
: Ca(OH)2 → CaO + H2O
Structure, preparation, occurrence
 Calcium hydroxide adopts a polymeric structure, as do all metal hydroxides. The structure is identical to that of Mg(OH) (''brucite structure''); i.e., the cadmium iodide motif. Strong
Calcium hydroxide adopts a polymeric structure, as do all metal hydroxides. The structure is identical to that of Mg(OH) (''brucite structure''); i.e., the cadmium iodide motif. Strong hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
s exist between the layers.
Calcium hydroxide is produced commercially by treating (slaking) lime with water:
:CaO + H2O → Ca(OH)2
In the laboratory it can be prepared by mixing aqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
solutions of calcium chloride
Calcium chloride is an inorganic compound, a salt with the chemical formula . It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide.
Ca ...
and sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and al ...
. The mineral form, portlandite
Portlandite is a hydroxide-bearing mineral typically included in the oxide mineral class. It is the naturally occurring form of calcium hydroxide (Ca(OH)2) and the calcium analogue of brucite (Mg(OH)2).
Occurrence
Portlandite occurs in a variety ...
, is relatively rare but can be found in some volcanic, plutonic, and metamorphic rocks
Metamorphic rocks arise from the transformation of existing rock to new types of rock in a process called metamorphism. The original rock (protolith) is subjected to temperatures greater than and, often, elevated pressure of or more, caus ...
. It has also been known to arise in burning coal dumps.
The positively charged ionized species CaOH+ has been detected in the atmosphere of S-type star
An S-type star (or just S star) is a cool giant with approximately equal quantities of carbon and oxygen in its atmosphere. The class was originally defined in 1922 by Paul Merrill for stars with unusual absorption lines and molecular bands no ...
s.
Retrograde solubility
According to Hopkins and Wulff (1965), the decrease of calcium hydroxide solubility with temperature was known since the works ofMarcellin Berthelot
Pierre Eugène Marcellin Berthelot (; 25 October 1827 – 18 March 1907) was a French chemist and Republican politician noted for the ThomsenBerthelot principle of thermochemistry. He synthesized many organic compounds from inorganic substa ...
(1875)Berthelot, M. (1875). Dissolution des acides et des alcalis. issolution of acids and alkalis In: Annales de Chimie et de Physique. Vol. 4, pp. 445–536. and Julius Thomsen
The gens Julia (''gēns Iūlia'', ) was one of the most prominent patrician families in ancient Rome. Members of the gens attained the highest dignities of the state in the earliest times of the Republic. The first of the family to obtain t ...
(1883)Thomsen J. (1883). Thermochemische untersuchungen hermochemical studies Vol. III, Johann Ambrosius Barth Verlag, Leipzig. (see Thomsen–Berthelot principle), when the presence of ions in aqueous solutions was still questioned. Since, it has been studied in detail by many authors, a.o., Miller and Witt (1929) or Johnston and Grove (1931) and refined many times (''e.g.'', Greenberg and Copeland (1960); Hopkins and Wulff (1965); Seewald and Seyfried (1991); Duchesne and Reardon (1995)).
The reason for this rather uncommon behavior is that the dissolution of calcium hydroxide in water is an exothermic process. Thus, according to Le Chatelier's principle
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French ...
, a lowering of temperature favours the elimination of the heat liberated through the process of dissolution and increases the equilibrium constant of dissolution of Ca(OH)2, and so increases its solubility at low temperature. This counter-intuitive temperature dependence of the solubility is referred to as "retrograde" or "inverse" solubility. The variably hydrated phases of calcium sulfate
Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris ...
(gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywa ...
, bassanite and anhydrite
Anhydrite, or anhydrous calcium sulfate, is a mineral with the chemical formula CaSO4. It is in the orthorhombic crystal system, with three directions of perfect cleavage parallel to the three planes of symmetry. It is not isomorphous with the ...
) also exhibit a retrograde solubility for the same reason because their dissolution reactions are exothermic.
Uses
Calcium hydroxide is commonly used to prepare lime mortar. One significant application of calcium hydroxide is as a flocculant, in water andsewage treatment
Sewage treatment (or domestic wastewater treatment, municipal wastewater treatment) is a type of wastewater treatment which aims to remove contaminants from sewage to produce an effluent that is suitable for discharge to the surrounding en ...
. It forms a fluffy charged solid that aids in the removal of smaller particles from water, resulting in a clearer product. This application is enabled by the low cost and low toxicity of calcium hydroxide. It is also used in fresh-water treatment for raising the pH of the water so that pipes will not corrode where the base water is acidic, because it is self-regulating and does not raise the pH too much.
It is also used in the preparation of ammonia gas (NH3), using the following reaction:
: Ca(OH)2 + 2 NH4Cl → 2 NH3 + CaCl2 + 2 H2O
Another large application is in the paper industry, where it is an intermediate in the reaction in the production of sodium hydroxide. This conversion is part of the ''causticizing'' step in the Kraft process
The kraft process (also known as kraft pulping or sulfate process) is a process for conversion of wood into wood pulp, which consists of almost pure cellulose fibres, the main component of paper. The kraft process involves treatment of wood chip ...
for making pulp.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . In the causticizing operation, burned lime is added to '' green liquor'', which is a solution primarily of sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
and sodium sulfate
Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 mil ...
produced by dissolving ''smelt'', which is the molten form of these chemicals from the recovery furnace.
In orchard
An orchard is an intentional plantation of trees or shrubs that is maintained for food production. Orchards comprise fruit- or nut-producing trees which are generally grown for commercial production. Orchards are also sometimes a feature of ...
crops, calcium hydroxide is used as a fungicide. Applications of 'lime water' prevent the development of cankers caused by the fungal pathogen ''Neonectria galligena
''Neonectria ditissima'' (syn. ''Neonectria galligena'') is a fungal plant pathogen. It causes cankers that can kill branches of trees by choking them off. Apple and beech trees are two susceptible species.
Host range
''Neonectria ditissima' ...
''. The trees are sprayed when they are dormant in winter to prevent toxic burns from the highly reactive calcium hydroxide. This use is authorised in the European Union and the United Kingdom under Basic Substance regulations.
Calcium hydroxide is used in dentistry, primarily in the specialty of endodontics.
Food industry
Because of its lowtoxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
and the mildness of its basic properties, slaked lime is widely used in the food industry
The food industry is a complex, global network of diverse businesses that supplies most of the food consumed by the world's population. The food industry today has become highly diversified, with manufacturing ranging from small, traditional, ...
:
* In USDA certified food production in plants and livestock
* To clarify raw juice from sugarcane
Sugarcane or sugar cane is a species of (often hybrid) tall, perennial grass (in the genus '' Saccharum'', tribe Andropogoneae) that is used for sugar production. The plants are 2–6 m (6–20 ft) tall with stout, jointed, fibrous stalk ...
or sugar beet
A sugar beet is a plant whose root contains a high concentration of sucrose and which is grown commercially for sugar production. In plant breeding, it is known as the Altissima cultivar group of the common beet ('' Beta vulgaris''). Together ...
s in the sugar industry
The sugar industry subsumes the production, processing and marketing of sugars (mostly sucrose and fructose). Globally, most sugar is extracted from sugar cane (~80% predominantly in the tropics) and sugar beet (~ 20%, mostly in temperate cl ...
, (see carbonatation
Carbonatation is a chemical reaction in which calcium hydroxide reacts with carbon dioxide and forms insoluble calcium carbonate:
:Ca(OH)2CO2->CaCO3H_2O
The process of forming a carbonate is sometimes referred to as "carbonation", although t ...
)
* To process water for alcoholic beverages and soft drinks
* Pickle cucumbers and other foods
* To make Chinese century egg
Century eggs (), also known under a wide variety of names (see infobox), are a Chinese egg-based culinary dish made by preserving duck, chicken or quail eggs in a mixture of clay, ash, salt, quicklime, and rice hulls for several weeks to s ...
s
* In maize preparation: removes the cellulose hull of maize kernels (see nixtamalization
Nixtamalization () is a process for the preparation of corn, or other grain, in which the grain is soaked and cooked in an alkaline solution, usually limewater (but sometimes aqueous alkali metal carbonates), washed, and then hulled. The ter ...
)
* To clear a brine
Brine is a high-concentration solution of salt (NaCl) in water (H2O). In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of solutions used for ...
of carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate ...
s of calcium and magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
in the manufacture of salt for food and pharmaceutical uses
* In fortifying (Ca supplement) fruit drinks, such as orange juice, and infant formula
Infant formula, baby formula, or simply formula (American English); or baby milk, infant milk or first milk (British English), is a manufactured food designed and marketed for feeding to babies and infants under 12 months of age, usually prepar ...
* As a digestive aid (called Choona, used in India in '' paan'', a mixture of areca nuts, calcium hydroxide and a variety of seeds wrapped in betel
The betel (''Piper betle'') is a vine of the family Piperaceae, which includes pepper and kava. The betel plant is native to Southeast Asia. It is an evergreen, dioecious perennial, with glossy heart-shaped leaves and white catkins. Betel p ...
leaves)
* As a substitute for baking soda
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3� ...
in making '' papadam''
* In the removal of carbon dioxide from controlled atmosphere produce storage rooms
* In the preparation of mushroom growing substrates
Native American uses
 In Nahuatl, the language of the Aztecs, the word for calcium hydroxide is ''nextli''. In a process called ''
In Nahuatl, the language of the Aztecs, the word for calcium hydroxide is ''nextli''. In a process called ''nixtamalization
Nixtamalization () is a process for the preparation of corn, or other grain, in which the grain is soaked and cooked in an alkaline solution, usually limewater (but sometimes aqueous alkali metal carbonates), washed, and then hulled. The ter ...
'', maize is cooked with nextli to become , also known as hominy
Hominy (Spanish: maíz molido; literally meaning "milled corn") is a food produced from dried maize (corn) kernels that have been treated with an alkali, in a process called nixtamalization ( is the Nahuatl word for "hominy"). "Lye hominy" is a ...
. Nixtamalization significantly increases the bioavailability of niacin
Niacin, also known as nicotinic acid, is an organic compound and a form of vitamin B3, an essential human nutrient. It can be manufactured by plants and animals from the amino acid tryptophan. Niacin is obtained in the diet from a variet ...
(vitamin B3), and is also considered tastier and easier to digest. Nixtamal is often ground into a flour, known as ''masa
''Masa'' (or ''masa de maíz'') (; ) is a maize dough that comes from ground nixtamalized corn. It is used for making corn tortillas, '' gorditas'', ''tamales'', '' pupusas'', and many other Latin American dishes. It is dried and powdered into ...
'', which is used to make tortillas and tamales.
In chewing coca leaves
Coca is any of the four cultivated plants in the family Erythroxylaceae, native to western South America. Coca is known worldwide for its psychoactive alkaloid, cocaine.
The plant is grown as a cash crop in the Argentine Northwest, Bolivia, A ...
, calcium hydroxide is usually chewed alongside to keep the alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of simila ...
stimulant
Stimulants (also often referred to as psychostimulants or colloquially as uppers) is an overarching term that covers many drugs including those that increase activity of the central nervous system and the body, drugs that are pleasurable and inv ...
s chemically available for absorption
Absorption may refer to:
Chemistry and biology
*Absorption (biology), digestion
**Absorption (small intestine)
*Absorption (chemistry), diffusion of particles of gas or liquid into liquid or solid materials
*Absorption (skin), a route by which s ...
by the body. Similarly, Native Americans traditionally chewed tobacco leaves with calcium hydroxide derived from burnt mollusc shells to enhance the effects. It has also been used by some indigenous American tribes as an ingredient in ''yopo
''Anadenanthera peregrina'', also known as yopo, jopo, cohoba, parica or calcium tree, is a perennial tree of the genus ''Anadenanthera'' native to the Caribbean and South America. It grows up to tall, and has a horny bark. Its flowers grow ...
'', a psychedelic snuff prepared from the beans of some '' Anadenanthera'' species.
Asian uses
Calcium hydroxide is typically added to a bundle ofareca nut
''Areca'' is a genus of 51 species of palms in the family Arecaceae, found in humid tropical forests from the islands of the Philippines, Malaysia and India, across Southeast Asia to Melanesia. The generic name ''Areca'' is derived from a name ...
and betel
The betel (''Piper betle'') is a vine of the family Piperaceae, which includes pepper and kava. The betel plant is native to Southeast Asia. It is an evergreen, dioecious perennial, with glossy heart-shaped leaves and white catkins. Betel p ...
leaf called " paan" to keep the alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of simila ...
stimulant
Stimulants (also often referred to as psychostimulants or colloquially as uppers) is an overarching term that covers many drugs including those that increase activity of the central nervous system and the body, drugs that are pleasurable and inv ...
s chemically available to enter the bloodstream via sublingual
Sublingual (abbreviated SL), from the Latin for "under the tongue", refers to the pharmacological route of administration by which substances diffuse into the blood through tissues under the tongue.
The sublingual glands receive their primary ...
absorption.
It is used in making '' naswar'' (also known as ''nass'' or ''niswar''), a type of dipping tobacco made from fresh tobacco leaves, calcium hydroxide (''chuna'' or ''soon''), and wood ash. It is consumed most in the Pathan diaspora, Afghanistan
Afghanistan, officially the Islamic Emirate of Afghanistan,; prs, امارت اسلامی افغانستان is a landlocked country located at the crossroads of Central Asia and South Asia. Referred to as the Heart of Asia, it is borde ...
, Pakistan
Pakistan ( ur, ), officially the Islamic Republic of Pakistan ( ur, , label=none), is a country in South Asia. It is the world's List of countries and dependencies by population, fifth-most populous country, with a population of almost 24 ...
, India
India, officially the Republic of India (Hindi: ), is a country in South Asia. It is the List of countries and dependencies by area, seventh-largest country by area, the List of countries and dependencies by population, second-most populous ...
and Bangladesh
Bangladesh (}, ), officially the People's Republic of Bangladesh, is a country in South Asia. It is the eighth-most populous country in the world, with a population exceeding 165 million people in an area of . Bangladesh is among the mo ...
. Villagers also use calcium hydroxide to paint their mud houses in Afghanistan, Pakistan and India.
Health risks
Unprotected exposure to Ca(OH)2 can cause severe skin irritation, chemical burns, blindness, lung damage or rashes.See also
*Baralyme Baralyme is a mixture of 80% calcium hydroxide and 20% barium hydroxide compounds that is used as an alternative to soda lime to absorb the exhaled carbon dioxide in a closed circuit anesthetic system.Mosby's Medical Dictionary, 8th edition. 2009, E ...
(carbon dioxide absorbent)
* Cement
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel (aggregate) together. Cement mixe ...
* Lime mortar
* Lime plaster
Lime plaster is a type of plaster composed of sand, water, and lime, usually non-hydraulic hydrated lime (also known as slaked lime, high calcium lime or air lime). Ancient lime plaster often contained horse hair for reinforcement and pozzolan ...
* Plaster
Plaster is a building material used for the protective or decorative coating of walls and ceilings and for moulding and casting decorative elements. In English, "plaster" usually means a material used for the interiors of buildings, while "re ...
* Magnesium hydroxide (less alkaline due to a lower solubility product)
* Soda lime
Soda lime is a mixture of NaOH and CaO chemicals, used in granular form in closed breathing environments, such as general anaesthesia, submarines, rebreathers and recompression chambers, to remove carbon dioxide from breathing gases to prevent ...
(carbon dioxide absorbent)
* Whitewash
Whitewash, or calcimine, kalsomine, calsomine, or lime paint is a type of paint made from slaked lime (calcium hydroxide, Ca(OH)2) or chalk calcium carbonate, (CaCO3), sometimes known as "whiting". Various other additives are sometimes used.
...
References
External links
* *CDC – NIOSH Pocket Guide to Chemical Hazards – Calcium Hydroxide
*
MSDS Data Sheet
{{DEFAULTSORT:Calcium Hydroxide Building materials Calcium compounds Dental materials Hydroxides Inorganic compounds Intoxication E-number additives