Heat capacity or thermal capacity is a
physical property
A physical property is any property of a physical system that is measurable. The changes in the physical properties of a system can be used to describe its changes between momentary states. A quantifiable physical property is called ''physical ...
of
matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic pa ...
, defined as the amount of
heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
to be supplied to an object to produce a unit change in its
temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
. The
SI unit
The International System of Units, internationally known by the abbreviation SI (from French ), is the modern form of the metric system and the world's most widely used system of units of measurement, system of measurement. It is the only system ...
of heat capacity is
joule
The joule ( , or ; symbol: J) is the unit of energy in the International System of Units (SI). In terms of SI base units, one joule corresponds to one kilogram- metre squared per second squared One joule is equal to the amount of work d ...
per
kelvin
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By de ...
(J/K).
Heat capacity is an
extensive property. The corresponding
intensive property is the
specific heat capacity
In thermodynamics, the specific heat capacity (symbol ) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. It is also referred to as massic heat ...
, found by dividing the heat capacity of an object by its mass. Dividing the heat capacity by the amount of substance in
moles yields its
molar heat capacity. The
volumetric heat capacity measures the heat capacity per
volume
Volume is a measure of regions in three-dimensional space. It is often quantified numerically using SI derived units (such as the cubic metre and litre) or by various imperial or US customary units (such as the gallon, quart, cubic inch) ...
. In
architecture
Architecture is the art and technique of designing and building, as distinguished from the skills associated with construction. It is both the process and the product of sketching, conceiving, planning, designing, and construction, constructi ...
and
civil engineering
Civil engineering is a regulation and licensure in engineering, professional engineering discipline that deals with the design, construction, and maintenance of the physical and naturally built environment, including public works such as roads ...
, the heat capacity of a building is often referred to as its ''
thermal mass''.
Definition
Basic definition
The heat capacity of an object, denoted by
, is the limit
where
is the amount of heat that must be added to the object (of mass ''M'') in order to raise its temperature by
.
The value of this parameter usually varies considerably depending on the starting temperature
of the object and the pressure
applied to it. In particular, it typically varies dramatically with
phase transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...
s such as melting or vaporization (see
enthalpy of fusion and
enthalpy of vaporization). Therefore, it should be considered a function
of those two variables.
Variation with temperature
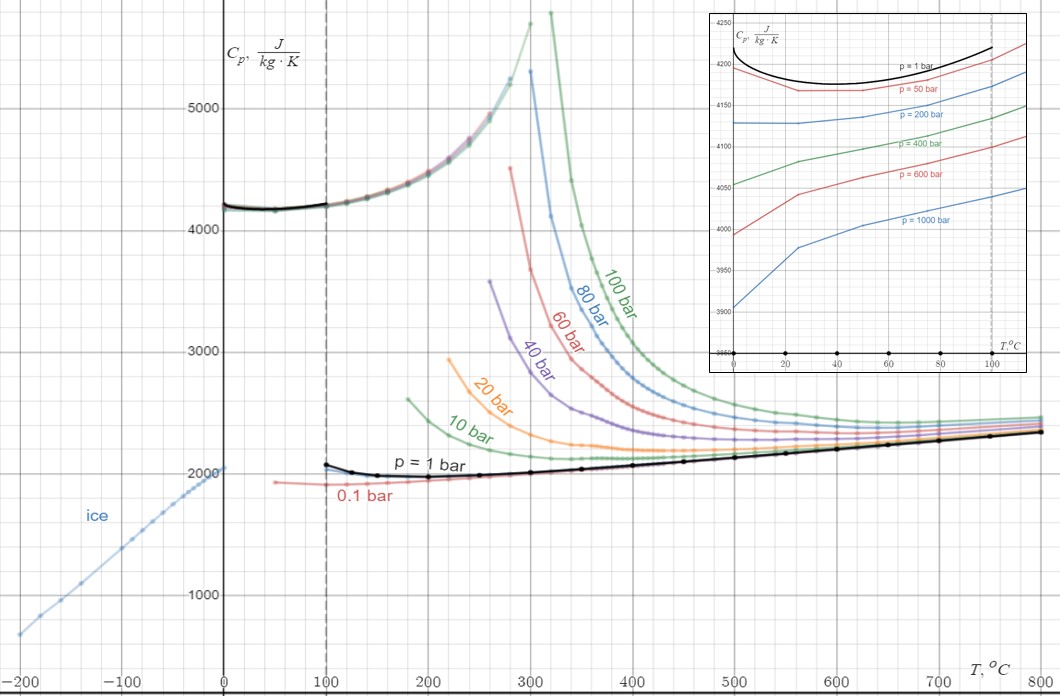
The variation can be ignored in contexts when working with objects in narrow ranges of temperature and pressure. For example, the heat capacity of a block of
iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
weighing one
pound is about 204 J/K when measured from a starting temperature ''T'' = 25 °C and ''P'' = 1 atm of pressure. That approximate value is adequate for temperatures between 15 °C and 35 °C, and surrounding pressures from 0 to 10 atmospheres, because the exact value varies very little in those ranges. One can trust that the same heat input of 204 J will raise the temperature of the block from 15 °C to 16 °C, or from 34 °C to 35 °C, with negligible error.
Heat capacities of a homogeneous system undergoing different thermodynamic processes
At constant pressure, ''δQ'' = ''dU'' + ''pdV'' ( isobaric process)
At constant pressure, heat supplied to the system contributes to both the
work done and the change in
internal energy
The internal energy of a thermodynamic system is the energy of the system as a state function, measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest, accoun ...
, according to the
first law of thermodynamics
The first law of thermodynamics is a formulation of the law of conservation of energy in the context of thermodynamic processes. For a thermodynamic process affecting a thermodynamic system without transfer of matter, the law distinguishes two ...
. The heat capacity is called
and defined as:
From the
first law of thermodynamics
The first law of thermodynamics is a formulation of the law of conservation of energy in the context of thermodynamic processes. For a thermodynamic process affecting a thermodynamic system without transfer of matter, the law distinguishes two ...
follows
and the inner energy as a function of
and
is:
 The variation can be ignored in contexts when working with objects in narrow ranges of temperature and pressure. For example, the heat capacity of a block of
The variation can be ignored in contexts when working with objects in narrow ranges of temperature and pressure. For example, the heat capacity of a block of  The variation can be ignored in contexts when working with objects in narrow ranges of temperature and pressure. For example, the heat capacity of a block of
The variation can be ignored in contexts when working with objects in narrow ranges of temperature and pressure. For example, the heat capacity of a block of