Hexacarbonylchromium on:
[Wikipedia]
[Google]
[Amazon]
Chromium hexacarbonyl (
CrCl3 ->
Early work on methods included contributions from luminaries such as


National Pollutant Inventory - Chromium (III) and compounds fact sheet
{{metal carbonyls Carbonyl complexes Chromium complexes Organochromium compounds Octahedral compounds
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
name: hexacarbonylchromium) is a chromium(0) organometallic compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and ...
with the formula . It is a homoleptic
In inorganic chemistry, a homoleptic chemical compound is a metal compound with all ligands identical. The term uses the " homo-" prefix to indicate that something is the same for all. Any metal species which has more than one type of ligand is he ...
complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
, which means that all the ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s are identical. It is a colorless crystalline
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macrosc ...
air-stable solid, with a high vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indicat ...
.
Preparation
Like manymetal carbonyl
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against n ...
s, is generally prepared by "reductive carbonylation", which involves reduction of a metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
with under an atmosphere of carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
. As described in a 2023 survey of methods "most cost-effective routes for the synthesis of group 6 Group 6 may refer to:
* Group 6 element, chemical element classification
* Group 6 (motorsport), FIA classification for sports car racing
* Group 6 Rugby League, rugby league competition in New South Wales, Australia
{{disambig ...
hexacarbonyls are based on the reduction of the metal chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
s ( , or ) with magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
, zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
or aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
powders... under CO pressures".
:text
Text may refer to:
Written word
* Text (literary theory)
In literary theory, a text is any object that can be "read", whether this object is a work of literature, a street sign, an arrangement of buildings on a city block, or styles of clothi ...
\text] Cr(CO)6Walter Hieber
Walter Hieber (18 December 1895 – 29 November 1976) was an inorganic chemist, known as the father of metal carbonyl chemistry. He was born 18 December 1895 and died 29 November 1976. Hieber's father was Johannes Hieber, an influential evange ...
, his student Ernst Otto Fischer
Ernst Otto Fischer (; 10 November 1918 – 23 July 2007) was a German chemist who won the Nobel Prize for pioneering work in the area of organometallic chemistry.
Early life
He was born in Solln, a borough of Munich. His parents were Karl T. Fi ...
, and Giulio Natta
Giulio Natta (; 26 February 1903 – 2 May 1979) was an Italian chemical engineer and Nobel laureate. He won a Nobel Prize in Chemistry in 1963 with Karl Ziegler for work on high density polymers. He also received a Lomonosov Gold Medal in 19 ...
. Using specially produced chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
metal will react with CO gas to give directly, although the method is not used commercially.
Electronic structure and bonding
In chromium hexacarbonyl, theoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
for chromium is assigned as zero, because Cr-C bonding electrons come from the C atom and are still assigned to C in the hypothetical ionic bond which determines the oxidation states. The formula conforms to the 18-electron rule and the complex adopts octahedral geometry with six carbonyl ligands.
The bonding between d6 chromium metal and neutral carbonyl ligands is described by the Dewar-Chatt-Duncanson model.It involves donation of electrons in HOMO of CO to empty d orbitals of the Cr metals while back-bonding from other d orbitals to the pi* orbital of the ligands reinforces the interactions synergistically.
The crystallographic studies on this compound have discovered the Cr–C and C–O distances of 1.916 and 1.171 Å, respectively. On one hand, there has been continuous efforts to calculate the electronic structures (including HOMO and LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
) as well as its molecular geometry on the chromium hexacarbonyl compound with various approaches. According to one of the most recent studies, the ground state configuration of turns out (2t2g)6(9 t1u)0(2t2u)0.
Reactions and applications
Photochemical reactions
Pentacarbonyl derivatives
When heated or UV-irradiated intetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
(THF) solution, converts to with loss of one CO ligand. The THF ligand is readily displaced. Often the THF complex is generated and used in situ.
UV-irradiation of frozen solutions of chromium hexacarbonyl affords a variety of labile adducts, including labile but complexes with some noble gases.
Photodimerization of norbornadiene
Norbornadiene was dimerized photochemically in the presence of , similarly to other metal complexes like , , and .Arene derivatives
Heating a solution of in anaromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
solvent results in replacement of three CO ligands. The reactions are especially favorable for electron-rich arenes:
:
The products are "piano stool complex
Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples in ...
es". These species are typically yellow solids. One example is (benzene)chromium tricarbonyl
(Benzene)chromium tricarbonyl is an organometallic compound with the formula . This yellow crystalline solid compound is soluble in common nonpolar organic solvents. The molecule adopts a geometry known as " piano stool" because of the planar ar ...
.
Fischer carbenes
Alkyl and arylorganolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s (RLi) add to to give anionic acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group () or hydrogen in the case of formyl grou ...
complexes. These anionic species in turn react with alkylating agent Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting ...
s such as trimethyloxonium tetrafluoroborate
Trimethyloxonium tetrafluoroborate is the organic compound with the formula . (It is sometimes called "Meerwein's salt" after Hans Meerwein.) This salt is a strong methylating agent, being a synthetic equivalent of . It is a white solid that rap ...
to form , where R stands for alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
, to give Fischer carbene
A Fischer carbene is a type of transition metal carbene complex, which is an organometallic compound containing a divalent organic ligand. In a Fischer carbene, the carbene ligand is a σ-donor π-acceptor ligand. Because π-backdonation from the ...
complexes:
:Cyclopentadienyl derivatives
Treatment of chromium hexacarbonyl withsodium cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pin ...
gives . Oxidation of this salt affords cyclopentadienylchromium tricarbonyl dimer
Cyclopentadienylchromium tricarbonyl dimer is the organochromium compound with the formula Cp2Cr2(CO)6, where Cp is C5H5. A dark green crystalline solid. It is the subject of research it exists in measureable equilibrium quantities with the mono ...
(). This complex is distinctive because it exists in measurable equilibrium with the monometallic Cr(I) radical .
Ligand-transfer reactions
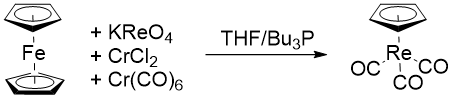
A unique double ligand-transfer reaction was reported with using chromium trichloride and chromium hexacarbonyl. In reactions,potassium perrhenate
Potassium perrhenate is an inorganic compound with the chemical formula KReO4.
Preparation
Potassium perrhenate can be produced by the neutralization of potassium hydroxide and perrhenic acid.
:\mathrm
Properties
Potassium perrhenate is a whi ...
() is reduced and carbonylated by the chromium reagents and undergoes ligand-transfer to afford complex derivatives.

Reduction
Chromium hexacarbonyl reacts with sodium electride in ammonia to give sodium pentacarbonylchromate(-II) and sodium acetylenediolate.Safety
In common with many of the other homoleptic metal carbonyls (e.g.nickel carbonyl
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a Organonickel chemistry, nickel(0) organometallic compound with the chemical formula, formula Ni(CO)4. This colorless liquid is the principal metal carbonyl, carbonyl of nickel. It is an React ...
and iron carbonyl There are three homoleptic iron carbonyl compounds:
* The monomeric iron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula . Under standard conditions Fe( CO)5 is a free-flowing, straw-colored liquid wit ...
), chromium hexacarbonyl is toxic and thought to be carcinogenic
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and Biological agent, biologic agent ...
. Its vapor pressure is relatively high for a metal complex, at 36 °C.
Historic literature
* * * * *References
External links
National Pollutant Inventory - Chromium (III) and compounds fact sheet
{{metal carbonyls Carbonyl complexes Chromium complexes Organochromium compounds Octahedral compounds