Heteronuclear Single Quantum Coherence Spectroscopy on:
[Wikipedia]
[Google]
[Amazon]
The heteronuclear single quantum coherence or heteronuclear single quantum correlation experiment, normally abbreviated as HSQC, is used frequently in NMR spectroscopy of organic molecules and is of particular significance in the field of
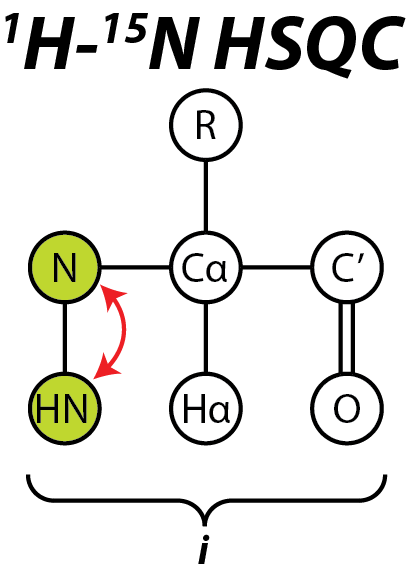 The 15N HSQC experiment is one of the most frequently recorded experiments in protein NMR. The HSQC experiment can be performed using the natural abundance of the 15N
The 15N HSQC experiment is one of the most frequently recorded experiments in protein NMR. The HSQC experiment can be performed using the natural abundance of the 15N
Protein NMR
Protein NMR spectra Biochemistry methods Spectroscopy Protein structure Nuclear magnetic resonance experiments
protein NMR Nuclear magnetic resonance spectroscopy of proteins (usually abbreviated protein NMR) is a field of structural biology in which NMR spectroscopy is used to obtain information about the structure and dynamics of proteins, and also nucleic acids, and ...
. The experiment was first described by Geoffrey Bodenhausen and D. J. Ruben in 1980. The resulting spectrum is two-dimensional (2D) with one axis for proton (1H) and the other for a heteronucleus (an atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden experiments, Geiger–Marsden gold foil experiment. After th ...
other than a proton), which is usually 13C or 15N. The spectrum contains a peak for each unique proton attached to the heteronucleus being considered. The 2D HSQC can also be combined with other experiments in higher-dimensional NMR experiments, such as NOESY-HSQC or TOCSY-HSQC.
General scheme
The HSQC experiment is a highly sensitive 2D-NMR experiment and was first described in a 1H—15N system, but is also applicable to other nuclei such as 1H—13C and 1H—31P. The basic scheme of this experiment involves the transfer of magnetization on the proton to the second nucleus, which may be 15N, 13C or 31P, via anINEPT Insensitive nuclei enhancement by polarization transfer (INEPT) is a signal enhancement method used in NMR spectroscopy. It involves the transfer of nuclear spin polarization from spins with large Boltzmann population differences to nuclear spins of ...
(Insensitive nuclei enhanced by polarization transfer) step. After a time delay (''t''1), the magnetization is transferred back to the proton via a retro-INEPT step and the signal is then recorded. In HSQC, a series of experiments is recorded where the time delay ''t''1 is incremented. The 1H signal is detected in the directly measured dimension in each experiment, while the chemical shift
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a ...
of 15N or 13C is recorded in the indirect dimension which is formed from the series of experiments.
HSQC in protein NMR
1H—15N HSQC
 The 15N HSQC experiment is one of the most frequently recorded experiments in protein NMR. The HSQC experiment can be performed using the natural abundance of the 15N
The 15N HSQC experiment is one of the most frequently recorded experiments in protein NMR. The HSQC experiment can be performed using the natural abundance of the 15N isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass number ...
, but normally for protein NMR, isotopically labeled proteins are used. Such labelled proteins are usually produced by expressing
Expression may refer to:
Linguistics
* Expression (linguistics), a word, phrase, or sentence
* Fixed expression, a form of words with a specific meaning
* Idiom, a type of fixed expression
* Metaphorical expression, a particular word, phrase, ...
the protein in cells grown in 15N-labelled media.
Each residue of the protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respon ...
, with the exception of proline, has an amide proton attached to a nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seve ...
in the peptide bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 ( nitrogen number two) of another, along a peptide or protein c ...
. The HSQC provides the correlation between the nitrogen and amide proton, and each amide yields a peak in the HSQC spectra. Each residue (except proline) therefore can produce an observable peak in the spectra, although in practice not all the peaks are always seen due to a number of factors. Normally the N-terminal residue (which has an NH3+ group attached) is not readily observable due to exchange with solvent. In addition to the backbone amide resonances, sidechains with nitrogen-bound protons will also produce peaks.
In a typical HSQC spectrum, the NH2 peaks from the sidechains of asparagine
Asparagine (symbol Asn or N) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the depro ...
and glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
appear as doublets on the top right corner, and a smaller peak may appear on top of each peak due to deuterium exchange from the D2O normally added to an NMR sample, giving these sidechain peaks a distinctive appearance. The sidechain amine peaks from tryptophan are usually shifted downfield and appear near the bottom left corner. The backbone amide peaks of glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinog ...
normally appear near the top of the spectrum.
The 15N HSQC is normally the first heteronuclear spectrum acquired for the assignment of resonances where each amide peak is assigned to a particular residue in the protein. If the protein is folded, the peaks are usually well-dispersed, and most of the individual peaks can be distinguished. If there is a large cluster of severely overlapped peaks around the middle of the spectrum, that would indicate the presence of significant unstructured elements in the protein. In such cases where there are severe overlap of resonances the assignment of resonances in the spectra can be difficult. The assignment of the HSQC spectrum requires other experiments, ideally using triple resonance experiments with 15N and 13C-labelled proteins, that provide sequential connectivities between residues so that the resonances can be linked to particular residues and sequentially assigned. The assignment of the spectrum is essential for a meaningful interpretation of more advanced NMR experiments such as structure determination and relaxation analysis.
Chemicals labelled with 15N isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass number ...
are relatively inexpensive, and the 15N HSQC is a sensitive experiment whereby a spectrum can be acquired in a relatively short time, the 15N HSQC is therefore often used to screen candidates for their suitability for structure determination by NMR, as well as optimization of the sample conditions. The time-consuming process of structure determination is usually not undertaken until a good HSQC spectrum can be obtained. The HSQC experiment is also useful for detecting binding interface in protein-protein interaction, as well the interactions with ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
s such as drugs. By comparing the HSQC of the free protein with the one bound to the ligand, changes in the chemical shift
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a ...
s of some peaks may be observed, and these peaks are likely to lie on the binding surface where the binding perturbed their chemical shifts. The 15N HSQC may also be used in relaxation analysis in the studies of molecular dynamics of proteins, the determination of ionization constant, and other studies.
1H—13C HSQC
This experiment provides correlations between a carbon and its attached protons. The constant time (CT) version of 1H—13C HSQC is normally used as it circumvents the issue of splitting of signal due to homonuclear 13C—13C ''J'' couplings which reduces spectral resolution. The "constant time" refers to the entire evolution period between the two INEPT steps which is kept constant in this experiment. If this evolution period is set to be the inverse of theJ-coupling
In nuclear chemistry and nuclear physics, ''J''-couplings (also called spin-spin coupling or indirect dipole–dipole coupling) are mediated through chemical bonds connecting two spins. It is an indirect interaction between two nuclear spins that ...
constant, then the sign of the magnetization of those carbons with an odd number of aliphatic carbon attached will be opposite to those with an even number. For example, if the Cβ of leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- ...
appears as a positive peak (2 aliphatic carbons attached), then the Cγ (3 aliphatic carbons attached) and Cα (1 aliphatic carbons attached) would appear negative.
HSQC in lipid NMR
1H—31P HSQC
The use of 1H—31P HSQC is relatively uncommon in lipidomics, however use of 31P in lipidomics dates back to the 1990s. The use of this technique is limited with respect to mass spectrometry due to its requirement for much bigger sample size, however the combination of 1H—31P HSQC with mass spectrometry is regarded as a thorough approach to lipidomics and techniques for 'dual spectroscopy' are becoming available.See also
*Protein NMR Nuclear magnetic resonance spectroscopy of proteins (usually abbreviated protein NMR) is a field of structural biology in which NMR spectroscopy is used to obtain information about the structure and dynamics of proteins, and also nucleic acids, and ...
*Nuclear Magnetic Resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
*Protein structure
Protein structure is the molecular geometry, three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single ami ...
*Structural biology
Structural biology is a field that is many centuries old which, and as defined by the Journal of Structural Biology, deals with structural analysis of living material (formed, composed of, and/or maintained and refined by living cells) at every le ...
References
{{reflistGeneral references
* Protein NMR Spectroscopy : Principles and Practice (1995) John Cavanagh, Wayne J. Fairbrother, Arthur G. Palmer III, Nicholas J. Skelton, Academic PressExternal links
Protein NMR
Protein NMR spectra Biochemistry methods Spectroscopy Protein structure Nuclear magnetic resonance experiments