Glucose on:
[Wikipedia]
[Google]
[Amazon]
Glucose is a simple sugar with the
 Glucose is usually present in solid form as a monohydrate with a closed
Glucose is usually present in solid form as a monohydrate with a closed
 The other open-chain isomer -glucose similarly gives rise to four distinct cyclic forms of -glucose, each the mirror image of the corresponding -glucose.
The glucopyranose ring (α or β) can assume several non-planar shapes, analogous to the "chair" and "boat" conformations of cyclohexane. Similarly, the glucofuranose ring may assume several shapes, analogous to the "envelope" conformations of
The other open-chain isomer -glucose similarly gives rise to four distinct cyclic forms of -glucose, each the mirror image of the corresponding -glucose.
The glucopyranose ring (α or β) can assume several non-planar shapes, analogous to the "chair" and "boat" conformations of cyclohexane. Similarly, the glucofuranose ring may assume several shapes, analogous to the "envelope" conformations of
 Mutarotation consists of a temporary reversal of the ring-forming reaction, resulting in the open-chain form, followed by a reforming of the ring. The ring closure step may use a different group than the one recreated by the opening step (thus switching between pyranose and furanose forms), or the new hemiacetal group created on C-1 may have the same or opposite handedness as the original one (thus switching between the α and β forms). Thus, though the open-chain form is barely detectable in solution, it is an essential component of the equilibrium.
The open-chain form is thermodynamically unstable, and it spontaneously isomerizes to the cyclic forms. (Although the ring closure reaction could in theory create four- or three-atom rings, these would be highly strained, and are not observed in practice.) In solutions at room temperature, the four cyclic isomers interconvert over a time scale of hours, in a process called mutarotation. Starting from any proportions, the mixture converges to a stable ratio of α:β 36:64. The ratio would be α:β 11:89 if it were not for the influence of the
Mutarotation consists of a temporary reversal of the ring-forming reaction, resulting in the open-chain form, followed by a reforming of the ring. The ring closure step may use a different group than the one recreated by the opening step (thus switching between pyranose and furanose forms), or the new hemiacetal group created on C-1 may have the same or opposite handedness as the original one (thus switching between the α and β forms). Thus, though the open-chain form is barely detectable in solution, it is an essential component of the equilibrium.
The open-chain form is thermodynamically unstable, and it spontaneously isomerizes to the cyclic forms. (Although the ring closure reaction could in theory create four- or three-atom rings, these would be highly strained, and are not observed in practice.) In solutions at room temperature, the four cyclic isomers interconvert over a time scale of hours, in a process called mutarotation. Starting from any proportions, the mixture converges to a stable ratio of α:β 36:64. The ratio would be α:β 11:89 if it were not for the influence of the
 In humans, glucose is metabolized by glycolysis and the pentose phosphate pathway.H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn: ''Biochemie''. Pearson Studium; 4. aktualisierte Auflage 2008; ; p. 490–496. (german) Glycolysis is used by all living organisms,Brian K. Hall: ''Strickberger's Evolution.'' Jones & Bartlett Publishers, 2013, , p. 164. with small variations, and all organisms generate energy from the breakdown of monosaccharides. In the further course of the metabolism, it can be completely degraded via oxidative decarboxylation, the citric acid cycle (synonym ''Krebs cycle'') and the
In humans, glucose is metabolized by glycolysis and the pentose phosphate pathway.H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn: ''Biochemie''. Pearson Studium; 4. aktualisierte Auflage 2008; ; p. 490–496. (german) Glycolysis is used by all living organisms,Brian K. Hall: ''Strickberger's Evolution.'' Jones & Bartlett Publishers, 2013, , p. 164. with small variations, and all organisms generate energy from the breakdown of monosaccharides. In the further course of the metabolism, it can be completely degraded via oxidative decarboxylation, the citric acid cycle (synonym ''Krebs cycle'') and the
 Glucose is a ubiquitous fuel in
Glucose is a ubiquitous fuel in
 Individuals with diabetes or other conditions that result in
Individuals with diabetes or other conditions that result in
 Most dietary carbohydrates contain glucose, either as their only building block (as in the polysaccharides starch and glycogen), or together with another monosaccharide (as in the hetero-polysaccharides sucrose and lactose). Unbound glucose is one of the main ingredients of honey. Glucose is extremely abundant and has been isolated from a variety of natural sources across the world, including male cones of the coniferous tree ''Wollemia nobilis'' in Rome, the roots of ''Ilex asprella'' plants in China, and straws from rice in California.
The carbohydrate value is calculated in the USDA database and does not always correspond to the sum of the sugars, the starch, and the "dietary fiber".
Most dietary carbohydrates contain glucose, either as their only building block (as in the polysaccharides starch and glycogen), or together with another monosaccharide (as in the hetero-polysaccharides sucrose and lactose). Unbound glucose is one of the main ingredients of honey. Glucose is extremely abundant and has been isolated from a variety of natural sources across the world, including male cones of the coniferous tree ''Wollemia nobilis'' in Rome, the roots of ''Ilex asprella'' plants in China, and straws from rice in California.
The carbohydrate value is calculated in the USDA database and does not always correspond to the sum of the sugars, the starch, and the "dietary fiber".
 Glucose is mainly used for the production of fructose and of glucose-containing foods. In foods, it is used as a sweetener, humectant, to increase the volume and to create a softer mouthfeel.
Various sources of glucose, such as grape juice (for wine) or malt (for beer), are used for fermentation to ethanol during the production of alcoholic beverages. Most soft drinks in the US use HFCS-55 (with a fructose content of 55% in the dry mass), while most other HFCS-sweetened foods in the US use HFCS-42 (with a fructose content of 42% in the dry mass). In Mexico, on the other hand, soft drinks are sweetened by cane sugar, which has a higher sweetening power. In addition, glucose syrup is used, inter alia, in the production of confectionery such as candies, toffee and fondant.Steve T. Beckett: ''Beckett's Industrial Chocolate Manufacture and Use''. John Wiley & Sons, 2017, , p. 82. Typical chemical reactions of glucose when heated under water-free conditions are
Glucose is mainly used for the production of fructose and of glucose-containing foods. In foods, it is used as a sweetener, humectant, to increase the volume and to create a softer mouthfeel.
Various sources of glucose, such as grape juice (for wine) or malt (for beer), are used for fermentation to ethanol during the production of alcoholic beverages. Most soft drinks in the US use HFCS-55 (with a fructose content of 55% in the dry mass), while most other HFCS-sweetened foods in the US use HFCS-42 (with a fructose content of 42% in the dry mass). In Mexico, on the other hand, soft drinks are sweetened by cane sugar, which has a higher sweetening power. In addition, glucose syrup is used, inter alia, in the production of confectionery such as candies, toffee and fondant.Steve T. Beckett: ''Beckett's Industrial Chocolate Manufacture and Use''. John Wiley & Sons, 2017, , p. 82. Typical chemical reactions of glucose when heated under water-free conditions are
wayback=20100331071121 ''Anlagen zum Positionspapier der Fachgruppe Nuklearchemie''
, February 2000. where it is by far the most commonly used diagnostic agent.
molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
. Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants
Plants are predominantly photosynthetic eukaryotes of the kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all current definitions of Plantae exclude ...
and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell w ...
in cell walls, the most abundant carbohydrate in the world.
In energy metabolism
Bioenergetics is a field in biochemistry and cell biology that concerns energy flow through living systems. This is an active area of biological research that includes the study of the transformation of energy in living organisms and the study of ...
, glucose is the most important source of energy in all organism
In biology, an organism () is any living system that functions as an individual entity. All organisms are composed of cells (cell theory). Organisms are classified by taxonomy into groups such as multicellular animals, plants, and ...
s. Glucose for metabolism is stored as a polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
, in plants mainly as starch and amylopectin Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α-glucose units found in plants. It is one of the two components of starch, the other being amylose.
Plants store starch within specialized organelles called amyloplas ...
, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is -glucose, while -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Glucose is naturally occurring and is found in its free state in fruits and other parts of plants. In animals, glucose is released from the breakdown of glycogen in a process known as glycogenolysis.
Glucose, as intravenous sugar solution, is on the World Health Organization's List of Essential Medicines
The WHO Model List of Essential Medicines (aka Essential Medicines List or EML), published by the World Health Organization (WHO), contains the medications considered to be most effective and safe to meet the most important needs in a health s ...
. It is also on the list in combination with sodium chloride.
The name glucose is derived from Ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic p ...
(, "wine, must"), from (, "sweet"). The suffix " -ose" is a chemical classifier, denoting a sugar.
History
Glucose was first isolated from raisins in 1747 by the German chemist Andreas Marggraf. Glucose was discovered in grapes by another German chemist Johann Tobias Lowitzin 1792, and distinguished as being different from cane sugar ( sucrose). Glucose is the term coined by Jean Baptiste Dumas in 1838, which has prevailed in the chemical literature. Friedrich August Kekulé proposed the term dextrose (from theLatin
Latin (, or , ) is a classical language belonging to the Italic branch of the Indo-European languages. Latin was originally a dialect spoken in the lower Tiber area (then known as Latium) around present-day Rome, but through the power of the ...
, meaning "right"), because in aqueous solution of glucose, the plane of linearly polarized light is turned to the right. In contrast, -fructose (a ketohexose) and -glucose turn linearly polarized light to the left. The earlier notation according to the rotation of the plane of linearly polarized light (''d'' and ''l''-nomenclature) was later abandoned in favor of the - and -notation, which refers to the absolute configuration of the asymmetric center farthest from the carbonyl group, and in concordance with the configuration of - or -glyceraldehyde.John F. Robyt: ''Essentials of Carbohydrate Chemistry.'' Springer Science & Business Media, 2012, . p. 7.
Since glucose is a basic necessity of many organisms, a correct understanding of its chemical
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., w ...
makeup and structure contributed greatly to a general advancement in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
. This understanding occurred largely as a result of the investigations of Emil Fischer
Hermann Emil Louis Fischer (; 9 October 1852 – 15 July 1919) was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He also developed the Fischer projection, a symbolic way of draw ...
, a German chemist who received the 1902 Nobel Prize in Chemistry for his findings. The synthesis of glucose established the structure of organic material and consequently formed the first definitive validation of Jacobus Henricus van 't Hoff's theories of chemical kinetics and the arrangements of chemical bonds in carbon-bearing molecules. Between 1891 and 1894, Fischer established the stereochemical configuration of all the known sugars and correctly predicted the possible isomers, applying Van 't Hoff's theory of asymmetrical carbon atoms. The names initially referred to the natural substances. Their enantiomers were given the same name with the introduction of systematic nomenclatures, taking into account absolute stereochemistry (e.g. Fischer nomenclature, / nomenclature).
For the discovery of the metabolism of glucose Otto Meyerhof received the Nobel Prize in Physiology or Medicine in 1922. Hans von Euler-Chelpin was awarded the Nobel Prize in Chemistry along with Arthur Harden
Sir Arthur Harden, FRS (12 October 1865 – 17 June 1940) was a British biochemist. He shared the Nobel Prize in Chemistry in 1929 with Hans Karl August Simon von Euler-Chelpin for their investigations into the fermentation of sugar and ferment ...
in 1929 for their "research on the fermentation of sugar and their share of enzymes in this process". In 1947, Bernardo Houssay (for his discovery of the role of the pituitary gland in the metabolism of glucose and the derived carbohydrates) as well as Carl and Gerty Cori
Gerty Theresa Cori (; August 15, 1896 – October 26, 1957) was an Austro-Hungarian and American biochemist who in 1947 was the third woman to win a Nobel Prize in science, and the first woman to be awarded the Nobel Prize in Physiology or Me ...
(for their discovery of the conversion of glycogen from glucose) received the Nobel Prize in Physiology or Medicine. In 1970, Luis Leloir
Luis Federico Leloir (September 6, 1906 – December 2, 1987) was an Argentine physician and biochemist who received the 1970 Nobel Prize in Chemistry for his discovery of the metabolic pathways in lactose. Although born in France, Leloir r ...
was awarded the Nobel Prize in Chemistry for the discovery of glucose-derived sugar nucleotides in the biosynthesis of carbohydrates.
Chemical and physical properties
Glucose forms white or colorless solids that are highlysoluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
in water and acetic acid but poorly soluble in methanol and ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
. They melt at (''α'') and (''β''), and decompose
Decomposition or rot is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is ...
starting at with release of various volatile products, ultimately leaving a residue of carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
.Wenyue Kang and Zhijun Zhang (2020): "Selective Production of Acetic Acid via Catalytic Fast Pyrolysis of Hexoses over Potassium Salts", ''Catalysts'', volume 10, pages 502-515. Glucose has a pK value of 12.16 at in water.
With six carbon atoms, it is classed as a hexose
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.
Hexoses exist in two forms, open-chain or cyclic, that easily convert ...
, a subcategory of the monosaccharides. -Glucose is one of the sixteen aldohexose stereoisomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
s. The - isomer, -glucose, also known as ''dextrose'', occurs widely in nature, but the -isomer, -glucose, does not. Glucose can be obtained by hydrolysis of carbohydrates such as milk sugar ( lactose), cane sugar (sucrose), maltose, cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell w ...
, glycogen, etc. Dextrose is commonly commercially manufactured from cornstarch in the US and Japan, from potato and wheat starch in Europe, and from tapioca starch in tropical areas. The manufacturing process uses hydrolysis via pressurized steaming at controlled pH in a jet followed by further enzymatic depolymerization. Unbonded glucose is one of the main ingredients of honey.
Structure and nomenclature
 Glucose is usually present in solid form as a monohydrate with a closed
Glucose is usually present in solid form as a monohydrate with a closed pyran
In chemistry, pyran, or oxine, is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C5H6O. There are two isomers of pyran that differ ...
ring (dextrose hydrate). In aqueous solution, on the other hand, it is an open-chain to a small extent and is present predominantly as α- or β- pyranose, which interconvert. From aqueous solutions, the three known forms can be crystallized: α-glucopyranose, β-glucopyranose and β-glucopyranose hydrate. Glucose is a building block of the disaccharides lactose and sucrose (cane or beet sugar), of oligosaccharides such as raffinose and of polysaccharides such as starch, amylopectin Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α-glucose units found in plants. It is one of the two components of starch, the other being amylose.
Plants store starch within specialized organelles called amyloplas ...
, glycogen, and cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell w ...
. The glass transition temperature of glucose is and the Gordon–Taylor constant (an experimentally determined constant for the prediction of the glass transition temperature for different mass fractions of a mixture of two substances) is 4.5.Benjamin Caballero, Paul Finglas, Fidel Toldrá: ''Encyclopedia of Food and Health''. Academic Press (2016). , Volume 1, p. 76.
Open-chain form
The open-chain form of glucose makes up less than 0.02% of the glucose molecules in an aqueous solution. The rest is one of two cyclic hemiacetal forms. In its open-chain form, the glucose molecule has an open (as opposed to cyclic) unbranched backbone of six carbon atoms, where C-1 is part of an aldehyde group . Therefore, glucose is also classified as an aldose, or an aldohexose. The aldehyde group makes glucose areducing sugar
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reacti ...
giving a positive reaction with the Fehling test.
Cyclic forms
In solutions, the open-chain form of glucose (either "-" or "-") exists in equilibrium with several cyclic isomers, each containing a ring of carbons closed by one oxygen atom. In aqueous solution, however, more than 99% of glucose molecules exist as pyranose forms. The open-chain form is limited to about 0.25%, andfuranose
A furanose is a collective term for carbohydrates that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle furan, bu ...
forms exist in negligible amounts. The terms "glucose" and "-glucose" are generally used for these cyclic forms as well. The ring arises from the open-chain form by an intramolecular nucleophilic addition reaction between the aldehyde group (at C-1) and either the C-4 or C-5 hydroxyl group, forming a hemiacetal linkage, .
The reaction between C-1 and C-5 yields a six-membered heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
system called a pyranose, which is a monosaccharide sugar (hence "-ose") containing a derivatised pyran
In chemistry, pyran, or oxine, is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C5H6O. There are two isomers of pyran that differ ...
skeleton. The (much rarer) reaction between C-1 and C-4 yields a five-membered furanose ring, named after the cyclic ether furan. In either case, each carbon in the ring has one hydrogen and one hydroxyl attached, except for the last carbon (C-4 or C-5) where the hydroxyl is replaced by the remainder of the open molecule (which is or respectively).
The ring-closing reaction can give two products, denoted "α-" and "β-". When a glucopyranose molecule is drawn in the Haworth projection, the designation "α-" means that the hydroxyl group attached to C-1 and the group at C-5 lies on opposite sides of the ring's plane (a '' trans'' arrangement), while "β-" means that they are on the same side of the plane (a '' cis'' arrangement). Therefore, the open-chain isomer -glucose gives rise to four distinct cyclic isomers: α--glucopyranose, β--glucopyranose, α--glucofuranose, and β--glucofuranose. These five structures exist in equilibrium and interconvert, and the interconversion is much more rapid with acid catalysis.
 The other open-chain isomer -glucose similarly gives rise to four distinct cyclic forms of -glucose, each the mirror image of the corresponding -glucose.
The glucopyranose ring (α or β) can assume several non-planar shapes, analogous to the "chair" and "boat" conformations of cyclohexane. Similarly, the glucofuranose ring may assume several shapes, analogous to the "envelope" conformations of
The other open-chain isomer -glucose similarly gives rise to four distinct cyclic forms of -glucose, each the mirror image of the corresponding -glucose.
The glucopyranose ring (α or β) can assume several non-planar shapes, analogous to the "chair" and "boat" conformations of cyclohexane. Similarly, the glucofuranose ring may assume several shapes, analogous to the "envelope" conformations of cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occu ...
.
In the solid state, only the glucopyranose forms are observed.
Some derivatives of glucofuranose, such as 1,2-''O''-isopropylidene--glucofuranose are stable and can be obtained pure as crystalline solids. For example, reaction of α-D-glucose with ''para''-tolylboronic acid reforms the normal pyranose ring to yield the 4-fold ester α-D-glucofuranose-1,2:3,5-bis(''p''-tolylboronate).
Mutarotation
 Mutarotation consists of a temporary reversal of the ring-forming reaction, resulting in the open-chain form, followed by a reforming of the ring. The ring closure step may use a different group than the one recreated by the opening step (thus switching between pyranose and furanose forms), or the new hemiacetal group created on C-1 may have the same or opposite handedness as the original one (thus switching between the α and β forms). Thus, though the open-chain form is barely detectable in solution, it is an essential component of the equilibrium.
The open-chain form is thermodynamically unstable, and it spontaneously isomerizes to the cyclic forms. (Although the ring closure reaction could in theory create four- or three-atom rings, these would be highly strained, and are not observed in practice.) In solutions at room temperature, the four cyclic isomers interconvert over a time scale of hours, in a process called mutarotation. Starting from any proportions, the mixture converges to a stable ratio of α:β 36:64. The ratio would be α:β 11:89 if it were not for the influence of the
Mutarotation consists of a temporary reversal of the ring-forming reaction, resulting in the open-chain form, followed by a reforming of the ring. The ring closure step may use a different group than the one recreated by the opening step (thus switching between pyranose and furanose forms), or the new hemiacetal group created on C-1 may have the same or opposite handedness as the original one (thus switching between the α and β forms). Thus, though the open-chain form is barely detectable in solution, it is an essential component of the equilibrium.
The open-chain form is thermodynamically unstable, and it spontaneously isomerizes to the cyclic forms. (Although the ring closure reaction could in theory create four- or three-atom rings, these would be highly strained, and are not observed in practice.) In solutions at room temperature, the four cyclic isomers interconvert over a time scale of hours, in a process called mutarotation. Starting from any proportions, the mixture converges to a stable ratio of α:β 36:64. The ratio would be α:β 11:89 if it were not for the influence of the anomeric effect
In organic chemistry, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the ''axial'' orientation instea ...
. Mutarotation is considerably slower at temperatures close to .
Optical activity
Whether in water or the solid form, -(+)-glucose isdextrorotatory
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
, meaning it will rotate the direction of polarized light clockwise as seen looking toward the light source. The effect is due to the chirality of the molecules, and indeed the mirror-image isomer, -(−)-glucose, is levorotatory (rotates polarized light counterclockwise) by the same amount. The strength of the effect is different for each of the five tautomers.
Note that the - prefix does not refer directly to the optical properties of the compound. It indicates that the C-5 chiral centre has the same handedness as that of -glyceraldehyde (which was so labelled because it is dextrorotatory). The fact that -glucose is dextrorotatory is a combined effect of its four chiral centres, not just of C-5; and indeed some of the other -aldohexoses are levorotatory.
The conversion between the two anomers can be observed in a polarimeter since pure α--glucose has a specific rotation angle of +112.2° mL/(dm·g), pure β--glucose of +17.5° mL/(dm·g).Manfred Hesse, Herbert Meier, Bernd Zeeh, Stefan Bienz, Laurent Bigler, Thomas Fox: ''Spektroskopische Methoden in der organischen Chemie''. 8th revised Edition. Georg Thieme, 2011, , p. 34 (in German). When equilibrium has been reached after a certain time due to mutarotation, the angle of rotation is +52.7° mL/(dm·g). By adding acid or base, this transformation is much accelerated. The equilibration takes place via the open-chain aldehyde form.
Isomerisation
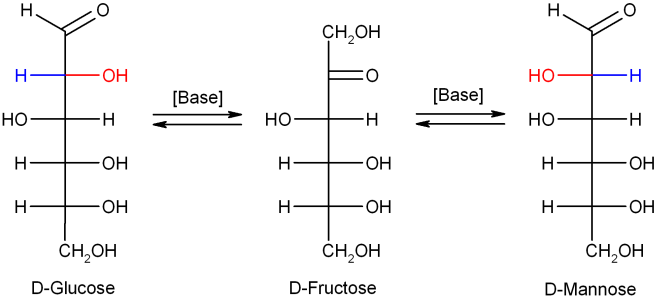
In dilute sodium hydroxide or other dilute bases, the monosaccharides mannose, glucose and fructose interconvert (via a Lobry de Bruyn–Alberda–Van Ekenstein transformation), so that a balance between these isomers is formed. This reaction proceeds via an enediol:
Biochemical properties
Glucose is the most abundant monosaccharide. Glucose is also the most widely used aldohexose in most living organisms. One possible explanation for this is that glucose has a lower tendency than other aldohexoses to react nonspecifically with theamine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
groups of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s. This reaction— glycation—impairs or destroys the function of many proteins, e.g. in glycated hemoglobin. Glucose's low rate of glycation can be attributed to its having a more stable cyclic form
Cyclic form is a technique of musical construction, involving multiple sections or movements, in which a theme, melody, or thematic material occurs in more than one movement as a unifying device. Sometimes a theme may occur at the beginning and ...
compared to other aldohexoses, which means it spends less time than they do in its reactive open-chain form. The reason for glucose having the most stable cyclic form of all the aldohexoses is that its hydroxy groups (with the exception of the hydroxy group on the anomeric carbon of -glucose) are in the equatorial position. Presumably, glucose is the most abundant natural monosaccharide because it is less glycated with proteins than other monosaccharides.Jeremy M. Berg: ''Stryer Biochemie.'' Springer-Verlag, 2017, , p. 531. (german) Another hypothesis is that glucose, being the only -aldohexose that has all five hydroxy substituents in the equatorial position in the form of β--glucose, is more readily accessible to chemical reactions, for example, for esterification
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
or acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments n ...
formation. For this reason, -glucose is also a highly preferred building block in natural polysaccharides (glycans). Polysaccharides that are composed solely of glucose are termed glucans.
Glucose is produced by plants through photosynthesis using sunlight, water and carbon dioxide and can be used by all living organisms as an energy and carbon source. However, most glucose does not occur in its free form, but in the form of its polymers, i.e. lactose, sucrose, starch and others which are energy reserve substances, and cellulose and chitin, which are components of the cell wall in plants or fungi
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately from ...
and arthropod
Arthropods (, (gen. ποδός)) are invertebrate animals with an exoskeleton, a segmented body, and paired jointed appendages. Arthropods form the phylum Arthropoda. They are distinguished by their jointed limbs and cuticle made of chiti ...
s, respectively. These polymers, when consumed by animals, fungi and bacteria, are degraded to glucose using enzymes. All animals are also able to produce glucose themselves from certain precursors as the need arises. Neuron
A neuron, neurone, or nerve cell is an electrically excitable cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous tissue in all animals except sponges and placozoa. ...
s, cells of the renal medulla
The renal medulla is the innermost part of the kidney. The renal medulla is split up into a number of sections, known as the renal pyramids. Blood enters into the kidney via the renal artery, which then splits up to form the segmental arteries whi ...
and erythrocytes
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "holl ...
depend on glucose for their energy production.Peter C. Heinrich: ''Löffler/Petrides Biochemie und Pathobiochemie.'' Springer-Verlag, 2014, , p. 195. (german) In adult humans, there is about of glucose,U. Satyanarayana: ''Biochemistry.'' Elsevier Health Sciences, 2014, . p. 674. of which about is present in the blood. Approximately of glucose is produced in the liver of an adult in 24 hours.
Many of the long-term complications of diabetes (e.g., blindness, kidney failure, and peripheral neuropathy
Peripheral neuropathy, often shortened to neuropathy, is a general term describing disease affecting the peripheral nerves, meaning nerves beyond the brain and spinal cord. Damage to peripheral nerves may impair sensation, movement, gland, or or ...
) are probably due to the glycation of proteins or lipids. In contrast, enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
-regulated addition of sugars to protein is called glycosylation and is essential for the function of many proteins.
Uptake
Ingested glucose initially binds to the receptor for sweet taste on the tongue in humans. This complex of the proteins T1R2 and T1R3 makes it possible to identify glucose-containing food sources. Glucose mainly comes from food—about per day is produced by conversion of food,Peter C. Heinrich: ''Löffler/Petrides Biochemie und Pathobiochemie.'' Springer-Verlag, 2014, , p. 404. but it is also synthesized from other metabolites in the body's cells. In humans, the breakdown of glucose-containing polysaccharides happens in part already duringchewing
Chewing or mastication is the process by which food is crushed and ground by teeth. It is the first step of digestion, and it increases the surface area of foods to allow a more efficient break down by enzymes. During the mastication process, th ...
by means of amylase
An amylase () is an enzyme that catalyses the hydrolysis of starch (Latin ') into sugars. Amylase is present in the saliva of humans and some other mammals, where it begins the chemical process of digestion. Foods that contain large amounts of ...
, which is contained in saliva, as well as by maltase
Maltase (, ''alpha-glucosidase'', ''glucoinvertase'', ''glucosidosucrase'', ''maltase-glucoamylase'', ''alpha-glucopyranosidase'', ''glucosidoinvertase'', ''alpha-D-glucosidase'', ''alpha-glucoside hydrolase'', ''alpha-1,4-glucosidase'', ''alp ...
, lactase
Lactase is an enzyme produced by many organisms. It is located in the brush border of the small intestine of humans and other mammals. Lactase is essential to the complete digestion of whole milk; it breaks down lactose, a sugar which gives ...
, and sucrase on the brush border of the small intestine. Glucose is a building block of many carbohydrates and can be split off from them using certain enzymes. Glucosidases, a subgroup of the glycosidases, first catalyze the hydrolysis of long-chain glucose-containing polysaccharides, removing terminal glucose. In turn, disaccharides are mostly degraded by specific glycosidases to glucose. The names of the degrading enzymes are often derived from the particular poly- and disaccharide; inter alia, for the degradation of polysaccharide chains there are amylases (named after amylose, a component of starch), cellulases (named after cellulose), chitinases (named after chitin), and more. Furthermore, for the cleavage of disaccharides, there are maltase, lactase, sucrase, trehalase
The enzyme Trehalase is a glycoside hydrolase, produced by cells in the brush border of the small intestine, which catalyzes the conversion of trehalose to glucose. It is found in most animals.
The non-reducing disaccharide trehalose (α-D-glucop ...
, and others. In humans, about 70 genes are known that code for glycosidases. They have functions in the digestion and degradation of glycogen, sphingolipid
Sphingolipids are a class of lipids containing a backbone of sphingoid bases, a set of aliphatic amino alcohols that includes sphingosine. They were discovered in brain extracts in the 1870s and were named after the mythological sphinx because o ...
s, mucopolysaccharides
Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units (i.e. two-sugar units). The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case o ...
, and poly(ADP-ribose
Adenosine diphosphate ribose (ADPR) is an ester molecule formed into chains by the enzyme poly ADP ribose polymerase. ADPR is created from cyclic ADP-ribose (cADPR) by the CD38 enzyme using nicotinamide adenine dinucleotide (NAD+) as a cofactor.
...
). Humans do not produce cellulases, chitinases, or trehalases, but the bacteria in the gut microbiota do.
In order to get into or out of cell membranes of cells and membranes of cell compartments, glucose requires special transport proteins from the major facilitator superfamily
The major facilitator superfamily (MFS) is a superfamily of membrane transport proteins that facilitate movement of small solutes across cell membranes in response to chemiosmotic gradients.
Function
The major facilitator superfamily (MFS) are ...
. In the small intestine (more precisely, in the jejunum
The jejunum is the second part of the small intestine in humans and most higher vertebrates, including mammals, reptiles, and birds. Its lining is specialised for the absorption by enterocytes of small nutrient molecules which have been previou ...
),Harold A. Harper: ''Medizinische Biochemie.'' Springer-Verlag, 2013, , p. 641. glucose is taken up into the intestinal epithelium with the help of glucose transporters via a secondary active transport
In cellular biology, ''active transport'' is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellul ...
mechanism called sodium ion-glucose symport
A symporter is an integral membrane protein that is involved in the transport of two (or more) different molecules across the cell membrane in the same direction. The symporter works in the plasma membrane and molecules are transported across the ...
via sodium/glucose cotransporter 1 (SGLT1). Further transfer occurs on the basolateral
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (th ...
side of the intestinal epithelial cells via the glucose transporter GLUT2, as well uptake into liver cells
A hepatocyte is a cell of the main parenchymal tissue of the liver. Hepatocytes make up 80% of the liver's mass.
These cells are involved in:
* Protein synthesis
* Protein storage
* Transformation of carbohydrates
* Synthesis of cholesterol, ...
, kidney cells, cells of the islets of Langerhans
The pancreatic islets or islets of Langerhans are the regions of the pancreas that contain its endocrine (hormone-producing) cells, discovered in 1869 by German pathological anatomist Paul Langerhans. The pancreatic islets constitute 1–2% of ...
, neuron
A neuron, neurone, or nerve cell is an electrically excitable cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous tissue in all animals except sponges and placozoa. ...
s, astrocyte
Astrocytes (from Ancient Greek , , "star" + , , "cavity", "cell"), also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of e ...
s, and tanycyte
Tanycytes are special ependymal cells found in the third ventricle of the brain, and on the floor of the fourth ventricle and have processes extending deep into the hypothalamus. It is possible that their function is to transfer chemical signals ...
s. Glucose enters the liver via the portal vein and is stored there as a cellular glycogen. In the liver cell, it is phosphorylated
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
by glucokinase at position 6 to form glucose 6-phosphate
Glucose 6-phosphate (G6P, sometimes called the Robison ester) is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this way ...
, which cannot leave the cell. Glucose 6-phosphatase can convert glucose 6-phosphate back into glucose exclusively in the liver, so the body can maintain a sufficient blood glucose concentration. In other cells, uptake happens by passive transport through one of the 14 GLUT proteins. In the other cell types, phosphorylation occurs through a hexokinase, whereupon glucose can no longer diffuse out of the cell.
The glucose transporter GLUT1
Glucose transporter 1 (or GLUT1), also known as solute carrier family 2, facilitated glucose transporter member 1 (SLC2A1), is a uniporter protein that in humans is encoded by the ''SLC2A1'' gene. GLUT1 facilitates the transport of glucose across ...
is produced by most cell types and is of particular importance for nerve cells and pancreatic β-cells. GLUT3 is highly expressed in nerve cells. Glucose from the bloodstream is taken up by GLUT4
Glucose transporter type 4 (GLUT4), also known as solute carrier family 2, facilitated glucose transporter member 4, is a protein encoded, in humans, by the ''SLC2A4'' gene. GLUT4 is the insulin-regulated glucose transporter found primarily in a ...
from muscle cells (of the skeletal muscle and heart muscle
Cardiac muscle (also called heart muscle, myocardium, cardiomyocytes and cardiac myocytes) is one of three types of vertebrate muscle tissues, with the other two being skeletal muscle and smooth muscle. It is an involuntary, striated muscle that ...
) and fat cells. GLUT14 is expressed exclusively in testicles. Excess glucose is broken down and converted into fatty acids, which are stored as triglycerides. In the kidneys, glucose in the urine is absorbed via SGLT1 and SGLT2
The sodium/glucose cotransporter 2 (SGLT2) is a protein that in humans is encoded by the (solute carrier family 5 (sodium/glucose cotransporter)) gene.
Function
SGLT2 is a member of the sodium glucose cotransporter family, which are sodium-d ...
in the apical cell membranes and transmitted via GLUT2 in the basolateral cell membranes. About 90% of kidney glucose reabsorption is via SGLT2 and about 3% via SGLT1.
Biosynthesis
In plants and some prokaryotes, glucose is a product of photosynthesis. Glucose is also formed by the breakdown of polymeric forms of glucose like glycogen (in animals and mushrooms) or starch (in plants). The cleavage of glycogen is termed glycogenolysis, the cleavage of starch is called starch degradation. The metabolic pathway that begins with molecules containing two to four carbon atoms (C) and ends in the glucose molecule containing six carbon atoms is called gluconeogenesis and occurs in all living organisms. The smaller starting materials are the result of other metabolic pathways. Ultimately almost all biomolecules come from the assimilation of carbon dioxide in plants and microbes during photosynthesis. The free energy of formation of α--glucose is 917.2 kilojoules per mole. In humans, gluconeogenesis occurs in the liver and kidney,Leszek Szablewski: ''Glucose Homeostasis and Insulin Resistance.'' Bentham Science Publishers, 2011, , p. 46. but also in other cell types. In the liver about of glycogen are stored, in skeletal muscle about .Peter C. Heinrich: ''Löffler/Petrides Biochemie und Pathobiochemie.'' Springer-Verlag, 2014, , p. 389. However, the glucose released in muscle cells upon cleavage of the glycogen can not be delivered to the circulation because glucose is phosphorylated by the hexokinase, and a glucose-6-phosphatase is not expressed to remove the phosphate group. Unlike for glucose, there is no transport protein for glucose-6-phosphate. Gluconeogenesis allows the organism to build up glucose from other metabolites, including lactate or certain amino acids, while consuming energy. The renal tubular cells can also produce glucose. Glucose also can be found outside of living organisms in the ambient environment. Glucose concentrations in the atmosphere are detected via collection of samples by aircraft and are known to vary from location to location. For example, glucose concentrations in atmospheric air from inland China range from 0.8-20.1 pg/L, whereas east coastal China glucose concentrations range from 10.3-142 pg/L.Glucose degradation
respiratory chain
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples thi ...
to water and carbon dioxide. If there is not enough oxygen available for this, the glucose degradation in animals occurs anaerobic to lactate via lactic acid fermentation and releases much less energy. Muscular lactate enters the liver through the bloodstream in mammals, where gluconeogenesis occurs ( Cori cycle). With a high supply of glucose, the metabolite acetyl-CoA from the Krebs cycle can also be used for fatty acid synthesis. Glucose is also used to replenish the body's glycogen stores, which are mainly found in liver and skeletal muscle. These processes are hormonally
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are required fo ...
regulated.
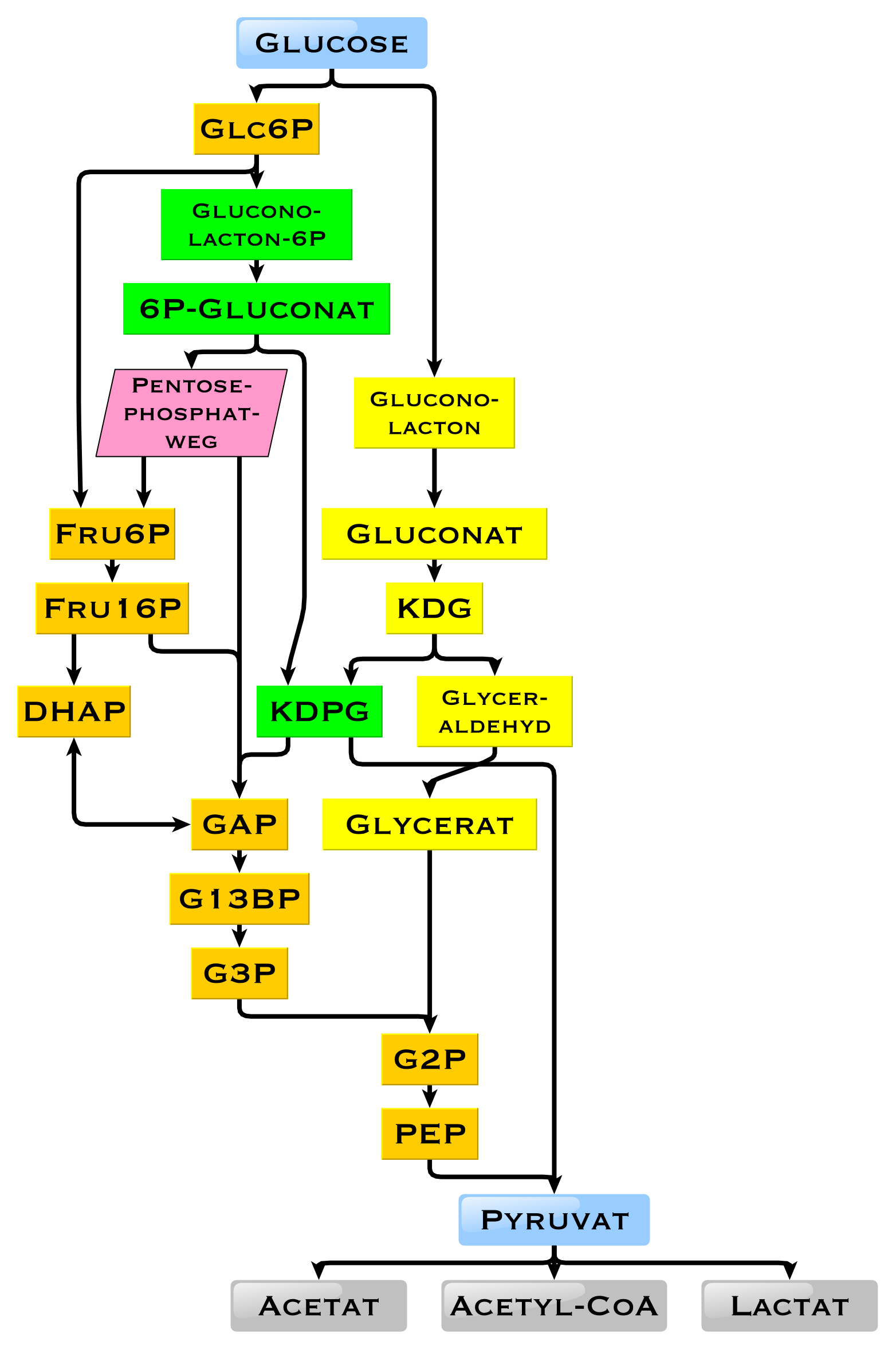
In other living organisms, other forms of fermentation can occur. The bacterium '' Escherichia coli'' can grow on nutrient media containing glucose as the sole carbon source. In some bacteria and, in modified form, also in archaea, glucose is degraded via the Entner-Doudoroff pathway.
Use of glucose as an energy source in cells is by either aerobic respiration, anaerobic respiration, or fermentation. The first step of glycolysis is the phosphorylation of glucose by a hexokinase to form glucose 6-phosphate
Glucose 6-phosphate (G6P, sometimes called the Robison ester) is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this way ...
. The main reason for the immediate phosphorylation of glucose is to prevent its diffusion out of the cell as the charged phosphate group prevents glucose 6-phosphate from easily crossing the cell membrane. Furthermore, addition of the high-energy phosphate group activates glucose for subsequent breakdown in later steps of glycolysis. At physiological conditions, this initial reaction is irreversible.
In anaerobic respiration, one glucose molecule produces a net gain of two ATP molecules (four ATP molecules are produced during glycolysis through substrate-level phosphorylation, but two are required by enzymes used during the process). In aerobic respiration, a molecule of glucose is much more profitable in that a maximum net production of 30 or 32 ATP molecules (depending on the organism) is generated,.
Tumor
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
cells often grow comparatively quickly and consume an above-average amount of glucose by glycolysis, which leads to the formation of lactate, the end product of fermentation in mammals, even in the presence of oxygen. This is called the Warburg effect. For the increased uptake of glucose in tumors various SGLT and GLUT are overly produced.
In yeast, ethanol is fermented at high glucose concentrations, even in the presence of oxygen (which normally leads to respiration rather than fermentation). This is called the Crabtree effect.
Glucose can also degrade to form carbon dioxide through abiotic means. This has been demonstrated to occur experimentally via oxidation and hydrolysis at 22˚C and a pH of 2.5.
Energy source
 Glucose is a ubiquitous fuel in
Glucose is a ubiquitous fuel in biology
Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up of cells that process hereditary i ...
. It is used as an energy source in organisms, from bacteria to humans, through either aerobic respiration, anaerobic respiration (in bacteria), or fermentation. Glucose is the human body's key source of energy, through aerobic respiration, providing about 3.75 kilocalories (16 kilojoule
The joule ( , ; symbol: J) is the unit of energy in the International System of Units (SI). It is equal to the amount of work done when a force of 1 newton displaces a mass through a distance of 1 metre in the direction of the force applied ...
s) of food energy
Food energy is chemical energy that animals (including humans) derive from their food to sustain their metabolism, including their muscular activity.
Most animals derive most of their energy from aerobic respiration, namely combining the carbohy ...
per gram. Breakdown of carbohydrates (e.g., starch) yields mono- and disaccharide
A disaccharide (also called a double sugar or ''biose'') is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, la ...
s, most of which is glucose. Through glycolysis and later in the reactions of the citric acid cycle and oxidative phosphorylation, glucose is oxidized to eventually form carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
and water, yielding energy mostly in the form of ATP. The insulin reaction, and other mechanisms, regulate the concentration of glucose in the blood. The physiological caloric value of glucose, depending on the source, is 16.2 kilojoules per gramGeorg Schwedt: ''Zuckersüße Chemie.'' John Wiley & Sons, 2012, , p. 100 . or 15.7 kJ/g (3.74 kcal/g). The high availability of carbohydrates from plant biomass has led to a variety of methods during evolution, especially in microorganisms, to utilize glucose for energy and carbon storage. Differences exist in which end product can no longer be used for energy production. The presence of individual genes, and their gene products, the enzymes, determine which reactions are possible. The metabolic pathway of glycolysis is used by almost all living beings. An essential difference in the use of glycolysis is the recovery of NADPH as a reductant for anabolism that would otherwise have to be generated indirectly.
Glucose and oxygen supply almost all the energy for the brain
A brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. It is located in the head, usually close to the sensory organs for senses such as vision. It is the most complex organ in a ve ...
, so its availability influences psychological processes. When glucose is low, psychological processes requiring mental effort (e.g., self-control, effortful decision-making) are impaired. In the brain, which is dependent on glucose and oxygen as the major source of energy, the glucose concentration is usually 4 to 6 mM (5 mM equals 90 mg/dL), but decreases to 2 to 3 mM when fasting. Confusion
In medicine, confusion is the quality or state of being bewildered or unclear. The term "acute mental confusion"
occurs below 1 mM and coma at lower levels.
The glucose in the blood is called blood sugar. Blood sugar levels are regulated by glucose-binding nerve cells in the hypothalamus
The hypothalamus () is a part of the brain that contains a number of small nuclei with a variety of functions. One of the most important functions is to link the nervous system to the endocrine system via the pituitary gland. The hypothalamu ...
. In addition, glucose in the brain binds to glucose receptors of the reward system in the nucleus accumbens. The binding of glucose to the sweet receptor on the tongue induces a release of various hormones of energy metabolism, either through glucose or through other sugars, leading to an increased cellular uptake and lower blood sugar levels. Artificial sweeteners do not lower blood sugar levels.
The blood sugar content of a healthy person in the short-time fasting state, e.g. after overnight fasting, is about 70 to 100 mg/dL of blood (4 to 5.5 mM). In blood plasma, the measured values are about 10–15% higher. In addition, the values in the arterial
An artery (plural arteries) () is a blood vessel in humans and most animals that takes blood away from the heart to one or more parts of the body (tissues, lungs, brain etc.). Most arteries carry oxygenated blood; the two exceptions are the pu ...
blood are higher than the concentrations in the venous
Veins are blood vessels in humans and most other animals that carry blood towards the heart. Most veins carry deoxygenated blood from the tissues back to the heart; exceptions are the pulmonary and umbilical veins, both of which carry oxygenated ...
blood since glucose is absorbed into the tissue during the passage of the capillary bed. Also in the capillary blood, which is often used for blood sugar determination, the values are sometimes higher than in the venous blood. The glucose content of the blood is regulated by the hormones insulin, incretin and glucagon. Insulin lowers the glucose level, glucagon increases it. Furthermore, the hormones adrenaline
Adrenaline, also known as epinephrine, is a hormone and medication which is involved in regulating visceral functions (e.g., respiration). It appears as a white microcrystalline granule. Adrenaline is normally produced by the adrenal glands an ...
, thyroxine
File:Thyroid_system.svg, upright=1.5, The thyroid system of the thyroid hormones T3 and T4
rect 376 268 820 433 Thyroid-stimulating hormone
rect 411 200 849 266 Thyrotropin-releasing hormone
rect 297 168 502 200 Hypothalamus
rect 66 216 386 25 ...
, glucocorticoids, somatotropin and adrenocorticotropin lead to an increase in the glucose level. There is also a hormone-independent regulation, which is referred to as glucose autoregulation. After food intake the blood sugar concentration increases. Values over 180 mg/dL in venous whole blood are pathological and are termed hyperglycemia, values below 40 mg/dL are termed hypoglycaemia. When needed, glucose is released into the bloodstream by glucose-6-phosphatase from glucose-6-phosphate originating from liver and kidney glycogen, thereby regulating the homeostasis
In biology, homeostasis (British also homoeostasis) (/hɒmɪə(ʊ)ˈsteɪsɪs/) is the state of steady internal, physical, and chemical conditions maintained by living systems. This is the condition of optimal functioning for the organism and ...
of blood glucose concentration. In ruminant
Ruminants (suborder Ruminantia) are hoofed herbivorous grazing or browsing mammals that are able to acquire nutrients from plant-based food by fermenting it in a specialized stomach prior to digestion, principally through microbial actions. The ...
s, the blood glucose concentration is lower (60 mg/dL in cattle and 40 mg/dL in sheep), because the carbohydrates are converted more by their gut microbiota into short-chain fatty acid Short-chain fatty acids (SCFAs) are fatty acids with fewer than six carbon atoms. Derived from intestinal microbial fermentation of indigestible foods, SCFAs are the main energy source of colonocytes, making them crucial to gastrointestinal healt ...
s.Harold A. Harper: ''Medizinische Biochemie''. Springer-Verlag, 2013, , p. 294.
Some glucose is converted to lactic acid by astrocyte
Astrocytes (from Ancient Greek , , "star" + , , "cavity", "cell"), also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of e ...
s, which is then utilized as an energy source by brain cells; some glucose is used by intestinal cells and red blood cell
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "holl ...
s, while the rest reaches the liver
The liver is a major organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it ...
, adipose tissue
Adipose tissue, body fat, or simply fat is a loose connective tissue composed mostly of adipocytes. In addition to adipocytes, adipose tissue contains the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular ...
and muscle cells, where it is absorbed and stored as glycogen (under the influence of insulin). Liver cell glycogen can be converted to glucose and returned to the blood when insulin is low or absent; muscle cell glycogen is not returned to the blood because of a lack of enzymes. In fat cells
Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Adipocytes are derived from mesenchymal stem cells which give rise to adipocytes through adipogenesis. I ...
, glucose is used to power reactions that synthesize some fat
In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds, most commonly those that occur in living beings or in food.
The term often refers specifically to triglycerides (triple est ...
types and have other purposes. Glycogen is the body's "glucose energy storage" mechanism, because it is much more "space efficient" and less reactive than glucose itself.
As a result of its importance in human health, glucose is an analyte in glucose tests that are common medical blood tests. Eating or fasting prior to taking a blood sample has an effect on analyses for glucose in the blood; a high fasting glucose blood sugar level may be a sign of prediabetes
Prediabetes is a component of the metabolic syndrome and is characterized by elevated blood sugar levels that fall below the threshold to diagnose diabetes mellitus. It usually does not cause symptoms but people with prediabetes often have obesit ...
or diabetes mellitus
Diabetes, also known as diabetes mellitus, is a group of metabolic disorders characterized by a high blood sugar level ( hyperglycemia) over a prolonged period of time. Symptoms often include frequent urination, increased thirst and increased ...
.
The glycemic index is an indicator of the speed of resorption and conversion to blood glucose levels from ingested carbohydrates, measured as the area under the curve
In mathematics, an integral assigns numbers to functions in a way that describes displacement, area, volume, and other concepts that arise by combining infinitesimal data. The process of finding integrals is called integration. Along with ...
of blood glucose levels after consumption in comparison to glucose (glucose is defined as 100).Richard A. Harvey, Denise R. Ferrier: ''Biochemistry''. 5th Edition, Lippincott Williams & Wilkins, 2011, , p. 366. The clinical importance of the glycemic index is controversial,U Satyanarayana: ''Biochemistry''. Elsevier Health Sciences, 2014, , p. 508. as foods with high fat contents slow the resorption of carbohydrates and lower the glycemic index, e.g. ice cream. An alternative indicator is the insulin index, measured as the impact of carbohydrate consumption on the blood insulin levels. The glycemic load
The glycemic load (GL) of food is a number that estimates how much the food will raise a person's blood glucose level after eating it. One unit of glycemic load approximates the effect of eating one gram of glucose. Glycemic load accounts for how ...
is an indicator for the amount of glucose added to blood glucose levels after consumption, based on the glycemic index and the amount of consumed food.
Precursor
Organisms use glucose as a precursor for the synthesis of several important substances. Starch,cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell w ...
, and glycogen ("animal starch") are common glucose polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s (polysaccharides). Some of these polymers (starch or glycogen) serve as energy stores, while others (cellulose and chitin, which is made from a derivative of glucose) have structural roles. Oligosaccharides of glucose combined with other sugars serve as important energy stores. These include lactose, the predominant sugar in milk, which is a glucose-galactose disaccharide, and sucrose, another disaccharide which is composed of glucose and fructose. Glucose is also added onto certain proteins and lipids in a process called glycosylation. This is often critical for their functioning. The enzymes that join glucose to other molecules usually use phosphorylated
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
glucose to power the formation of the new bond by coupling it with the breaking of the glucose-phosphate bond.
Other than its direct use as a monomer, glucose can be broken down to synthesize a wide variety of other biomolecules. This is important, as glucose serves both as a primary store of energy and as a source of organic carbon. Glucose can be broken down and converted into lipids. It is also a precursor for the synthesis of other important molecules such as vitamin C (ascorbic acid). In living organisms, glucose is converted to several other chemical compounds that are the starting material for various metabolic pathways. Among them, all other monosaccharidesPeter C. Heinrich: ''Löffler/Petrides Biochemie und Pathobiochemie.'' Springer-Verlag, 2014, , p. 27. such as fructose (via the polyol pathway The polyol pathway is a two-step process that converts glucose to fructose. In this pathway glucose is reduced to sorbitol, which is subsequently oxidized to fructose. It is also called the sorbitol-aldose reductase pathway.
The pathway is implic ...
),Peter C. Heinrich: ''Löffler/Petrides Biochemie und Pathobiochemie.'' Springer-Verlag, 2014, , p. 199, 200. mannose (the epimer of glucose at position 2), galactose (the epimer at position 4), fucose, various uronic acids and the amino sugars are produced from glucose.Peter C. Heinrich: ''Löffler/Petrides Biochemie und Pathobiochemie.'' Springer-Verlag, 2014, , p. 214. In addition to the phosphorylation to glucose-6-phosphate, which is part of the glycolysis, glucose can be oxidized during its degradation to glucono-1,5-lactone. Glucose is used in some bacteria as a building block in the trehalose
Trehalose (from Turkish '' tıgala'' – a sugar derived from insect cocoons + -ose) is a sugar consisting of two molecules of glucose. It is also known as mycose or tremalose. Some bacteria, fungi, plants and invertebrate animals synthesize it ...
or the dextran biosynthesis and in animals as a building block of glycogen. Glucose can also be converted from bacterial xylose isomerase to fructose. In addition, glucose metabolites produce all nonessential amino acids, sugar alcohol
Sugar alcohols (also called polyhydric alcohols, polyalcohols, alditols or glycitols) are organic compounds, typically derived from sugars, containing one hydroxyl group (–OH) attached to each carbon atom. They are white, water-soluble solids ...
s such as mannitol
Mannitol is a type of sugar alcohol used as a sweetener and medication. It is used as a low calorie sweetener as it is poorly absorbed by the intestines. As a medication, it is used to decrease pressure in the eyes, as in glaucoma, and to lo ...
and sorbitol, fatty acids, cholesterol and nucleic acids. Finally, glucose is used as a building block in the glycosylation of proteins to glycoproteins, glycolipids, peptidoglycan
Peptidoglycan or murein is a unique large macromolecule, a polysaccharide, consisting of sugars and amino acids that forms a mesh-like peptidoglycan layer outside the plasma membrane, the rigid cell wall (murein sacculus) characteristic of most ba ...
s, glycosides and other substances (catalyzed by glycosyltransferase
Glycosyltransferases (GTFs, Gtfs) are enzymes (EC 2.4) that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar (also known as the "glycosyl donor") to a nucleophilic glyco ...
s) and can be cleaved from them by glycosidases.
Pathology
Diabetes
Diabetes is a metabolic disorder where the body is unable to regulate levels of glucose in the blood either because of a lack of insulin in the body or the failure, by cells in the body, to respond properly to insulin. Each of these situations can be caused by persistently high elevations of blood glucose levels, through pancreatic burnout and insulin resistance. The pancreas is the organ responsible for the secretion of the hormones insulin and glucagon. Insulin is a hormone that regulates glucose levels, allowing the body's cells to absorb and use glucose. Without it, glucose cannot enter the cell and therefore cannot be used as fuel for the body's functions. If the pancreas is exposed to persistently high elevations of blood glucose levels, the insulin-producing cells in the pancreas could be damaged, causing a lack of insulin in the body. Insulin resistance occurs when the pancreas tries to produce more and more insulin in response to persistently elevated blood glucose levels. Eventually, the rest of the body becomes resistant to the insulin that the pancreas is producing, thereby requiring more insulin to achieve the same blood glucose-lowering effect, and forcing the pancreas to produce even more insulin to compete with the resistance. This negative spiral contributes to pancreatic burnout, and the disease progression of diabetes. To monitor the body's response to blood glucose-lowering therapy, glucose levels can be measured.Blood glucose monitoring
Blood glucose monitoring is the use of a glucose meter for testing the concentration of glucose in the blood ( glycemia). Particularly important in diabetes management, a blood glucose test is typically performed by piercing the skin (typically, ...
can be performed by multiple methods, such as the fasting glucose test which measures the level of glucose in the blood after 8 hours of fasting. Another test is the 2-hour glucose tolerance test (GTT)for this test, the person has a fasting glucose test done, then drinks a 75-gram glucose drink and is retested. This test measures the ability of the person's body to process glucose. Over time the blood glucose levels should decrease as insulin allows it to be taken up by cells and exit the blood stream.
Hypoglycemia management
 Individuals with diabetes or other conditions that result in
Individuals with diabetes or other conditions that result in low blood sugar
Hypoglycemia, also called low blood sugar, is a fall in blood sugar to levels below normal, typically below 70 mg/dL (3.9 mmol/L). Whipple's triad is used to properly identify hypoglycemic episodes. It is defined as blood glucose bel ...
often carry small amounts of sugar in various forms. One sugar commonly used is glucose, often in the form of glucose tablets (glucose pressed into a tablet shape sometimes with one or more other ingredients as a binder), hard candy, or sugar packet
A sugar packet is a delivery method for one serving of sugar or other sweetener. Sugar packets are commonly supplied in restaurants, coffeehouses, and tea houses, where they are preferred to sugar bowls or sugar dispensers for reasons of neatness, ...
.
Sources
 Most dietary carbohydrates contain glucose, either as their only building block (as in the polysaccharides starch and glycogen), or together with another monosaccharide (as in the hetero-polysaccharides sucrose and lactose). Unbound glucose is one of the main ingredients of honey. Glucose is extremely abundant and has been isolated from a variety of natural sources across the world, including male cones of the coniferous tree ''Wollemia nobilis'' in Rome, the roots of ''Ilex asprella'' plants in China, and straws from rice in California.
The carbohydrate value is calculated in the USDA database and does not always correspond to the sum of the sugars, the starch, and the "dietary fiber".
Most dietary carbohydrates contain glucose, either as their only building block (as in the polysaccharides starch and glycogen), or together with another monosaccharide (as in the hetero-polysaccharides sucrose and lactose). Unbound glucose is one of the main ingredients of honey. Glucose is extremely abundant and has been isolated from a variety of natural sources across the world, including male cones of the coniferous tree ''Wollemia nobilis'' in Rome, the roots of ''Ilex asprella'' plants in China, and straws from rice in California.
The carbohydrate value is calculated in the USDA database and does not always correspond to the sum of the sugars, the starch, and the "dietary fiber".
Commercial production
Glucose is produced industrially from starch by enzymatic hydrolysis using glucose amylase or by the use ofacids
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a ...
. Enzymatic hydrolysis has largely displaced acid-catalyzed hydrolysis reactions.P. J. Fellows: ''Food Processing Technology. Woodhead Publishing'', 2016, , p. 197. The result is glucose syrup (enzymatically with more than 90% glucose in the dry matter) with an annual worldwide production volume of 20 million tonnes (as of 2011).Thomas Becker, Dietmar Breithaupt, Horst Werner Doelle, Armin Fiechter, Günther Schlegel, Sakayu Shimizu, Hideaki Yamada: ''Biotechnology'', in: ''Ullmann's Encyclopedia of Industrial Chemistry'', 7th Edition, Wiley-VCH, 2011. . Volume 6, p. 48. This is the reason for the former common name "starch sugar". The amylases most often come from ''Bacillus licheniformis
''Bacillus licheniformis'' is a bacterium commonly found in the soil. It is found on bird feathers, especially chest and back plumage, and most often in ground-dwelling birds (like sparrows) and aquatic species (like ducks).
It is a gram-posi ...
''The Amylase Research Society of Japan: ''Handbook of Amylases and Related Enzymes.'' Elsevier, 2014, , p. 195. or ''Bacillus subtilis
''Bacillus subtilis'', known also as the hay bacillus or grass bacillus, is a Gram-positive, catalase-positive bacterium, found in soil and the gastrointestinal tract of ruminants, humans and marine sponges. As a member of the genus ''Bacillus ...
'' (strain MN-385), which are more thermostable than the originally used enzymes. Starting in 1982, pullulanases from ''Aspergillus niger
''Aspergillus niger'' is a mold classified within the ''Nigri'' section of the ''Aspergillus'' genus. The ''Aspergillus'' genus consists of common molds found throughout the environment within soil and water, on vegetation, in fecal matter, on de ...
'' were used in the production of glucose syrup to convert amylopectin to starch (amylose), thereby increasing the yield of glucose. The reaction is carried out at a pH = 4.6–5.2 and a temperature of 55–60 °C. Corn syrup has between 20% and 95% glucose in the dry matter. The Japanese form of the glucose syrup, Mizuame
is a sweetener from Japan. A clear, thick, sticky liquid, it is made by converting starch to sugars. is added to to give them a sheen, eaten in ways similar to honey, and can be a main ingredient in sweets. Some are produced in a very simil ...
, is made from sweet potato or rice
Rice is the seed of the grass species '' Oryza sativa'' (Asian rice) or less commonly ''Oryza glaberrima'' (African rice). The name wild rice is usually used for species of the genera '' Zizania'' and '' Porteresia'', both wild and domesticat ...
starch. Maltodextrin
Maltodextrin is a polysaccharide that is used as a food ingredient. It is produced from vegetable starch by partial hydrolysis and is usually found as a white hygroscopic spray-dried powder. Maltodextrin is easily digestible, being absorbed as ...
contains about 20% glucose.
Many crops can be used as the source of starch. Maize
Maize ( ; ''Zea mays'' subsp. ''mays'', from es, maíz after tnq, mahiz), also known as corn (North American and Australian English), is a cereal grain first domesticated by indigenous peoples in southern Mexico about 10,000 years ago. The ...
, rice, wheat
Wheat is a grass widely cultivated for its seed, a cereal grain that is a worldwide staple food. The many species of wheat together make up the genus ''Triticum'' ; the most widely grown is common wheat (''T. aestivum''). The archaeologi ...
, cassava, potato, barley, sweet potato,Alan Davidson: ''The Oxford Companion to Food''. OUP Oxford, 2014, , p. 527. corn husk
Husk (or hull) in botany is the outer shell or coating of a seed. In the United States, the term husk often refers to the leafy outer covering of an ear of maize (corn) as it grows on the plant. Literally, a husk or hull includes the protective ...
and sago
Sago () is a starch extracted from the pith, or spongy core tissue, of various tropical palm stems, especially those of ''Metroxylon sagu''. It is a major staple food for the lowland peoples of New Guinea and the Maluku Islands, where it is c ...
are all used in various parts of the world. In the United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territori ...
, corn starch (from maize) is used almost exclusively. Some commercial glucose occurs as a component of invert sugar, a roughly 1:1 mixture of glucose and fructose that is produced from sucrose. In principle, cellulose could be hydrolyzed to glucose, but this process is not yet commercially practical.
Conversion to fructose
In the US, almost exclusively corn (more precisely, corn syrup) is used as glucose source for the production of isoglucose, which is a mixture of glucose and fructose, since fructose has a higher sweetening powerwith same physiological calorific value of 374 kilocalories per 100 g. The annual world production of isoglucose is 8 million tonnes (as of 2011). When made from corn syrup, the final product ishigh-fructose corn syrup
High-fructose corn syrup (HFCS), also known as glucose–fructose, isoglucose and glucose–fructose syrup, is a sweetener made from corn starch. As in the production of conventional corn syrup, the starch is broken down into glucose by enzy ...
(HFCS).
Commercial usage
 Glucose is mainly used for the production of fructose and of glucose-containing foods. In foods, it is used as a sweetener, humectant, to increase the volume and to create a softer mouthfeel.
Various sources of glucose, such as grape juice (for wine) or malt (for beer), are used for fermentation to ethanol during the production of alcoholic beverages. Most soft drinks in the US use HFCS-55 (with a fructose content of 55% in the dry mass), while most other HFCS-sweetened foods in the US use HFCS-42 (with a fructose content of 42% in the dry mass). In Mexico, on the other hand, soft drinks are sweetened by cane sugar, which has a higher sweetening power. In addition, glucose syrup is used, inter alia, in the production of confectionery such as candies, toffee and fondant.Steve T. Beckett: ''Beckett's Industrial Chocolate Manufacture and Use''. John Wiley & Sons, 2017, , p. 82. Typical chemical reactions of glucose when heated under water-free conditions are
Glucose is mainly used for the production of fructose and of glucose-containing foods. In foods, it is used as a sweetener, humectant, to increase the volume and to create a softer mouthfeel.
Various sources of glucose, such as grape juice (for wine) or malt (for beer), are used for fermentation to ethanol during the production of alcoholic beverages. Most soft drinks in the US use HFCS-55 (with a fructose content of 55% in the dry mass), while most other HFCS-sweetened foods in the US use HFCS-42 (with a fructose content of 42% in the dry mass). In Mexico, on the other hand, soft drinks are sweetened by cane sugar, which has a higher sweetening power. In addition, glucose syrup is used, inter alia, in the production of confectionery such as candies, toffee and fondant.Steve T. Beckett: ''Beckett's Industrial Chocolate Manufacture and Use''. John Wiley & Sons, 2017, , p. 82. Typical chemical reactions of glucose when heated under water-free conditions are caramelization
Caramelization is a process of browning of sugar used extensively in cooking for the resulting sweet nutty flavor and brown color. The brown colors are produced by three groups of polymers: caramelans (C24H36O18), caramelens (C36H50O25), and ca ...
and, in presence of amino acids, the Maillard reaction.
In addition, various organic acids can be biotechnologically produced from glucose, for example by fermentation with '' Clostridium thermoaceticum'' to produce acetic acid, with ''Penicillium notatum
''Penicillium chrysogenum'' (formerly known as ''Penicillium notatum'') is a species of fungus in the genus ''Penicillium''. It is common in temperate and subtropical regions and can be found on salted food products, but it is mostly found in in ...
'' for the production of araboascorbic acid, with '' Rhizopus delemar'' for the production of fumaric acid
Fumaric acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297.
The salts and esters are known as fu ...
, with ''Aspergillus niger
''Aspergillus niger'' is a mold classified within the ''Nigri'' section of the ''Aspergillus'' genus. The ''Aspergillus'' genus consists of common molds found throughout the environment within soil and water, on vegetation, in fecal matter, on de ...
'' for the production of gluconic acid, with '' Candida brumptii'' to produce isocitric acid, with ''Aspergillus terreus
''Aspergillus terreus'', also known as ''Aspergillus terrestris'', is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, ''A. terreus'' is now known to be capable of sexual reproduction. This saprotr ...
'' for the production of itaconic acid, with '' Pseudomonas fluorescens'' for the production of 2-ketogluconic acid, with '' Gluconobacter suboxydans'' for the production of 5-ketogluconic acid, with '' Aspergillus oryzae'' for the production of kojic acid
Kojic acid is a chelation agent produced by several species of fungi, especially ''Aspergillus oryzae'', which has the Japanese common name ''koji''. Kojic acid is a by-product in the fermentation process of malting rice, for use in the manufactur ...
, with '' Lactobacillus delbrueckii'' for the production of lactic acid, with ''Lactobacillus brevis
''Levilactobacillus brevis'' is a gram-positive, rod shaped species of lactic acid bacteria which is heterofermentative, creating CO2, lactic acid and acetic acid or ethanol during fermentation. ''L. brevis'' is the type species of the genus ''Le ...
'' for the production of malic acid, with '' Propionibacter shermanii'' for the production of propionic acid, with '' Pseudomonas aeruginosa'' for the production of pyruvic acid and with '' Gluconobacter suboxydans'' for the production of tartaric acid.James A. Kent: ''Riegel's Handbook of Industrial Chemistry''. Springer Science & Business Media, 2013, , p. 938. Potent, bioactive natural products like triptolide that inhibit mammalian transcription via inhibition of the XPB subunit of the general transcription factor TFIIH has been recently reported as a glucose conjugate for targeting hypoxic cancer cells with increased glucose transporter expression. Recently, glucose has been gaining commercial use as a key component of "kits" containing lactic acid and insulin intended to induce hypoglycemia and hyperlactatemia to combat different cancers and infections.
Analysis
When a glucose molecule is to be detected at a certain position in a larger molecule,nuclear magnetic resonance spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fie ...
, X-ray crystallography analysis or lectin immunostaining is performed with concanavalin A
Concanavalin A (ConA) is a lectin (carbohydrate-binding protein) originally extracted from the jack-bean (''Canavalia ensiformis''). It is a member of the legume lectin family. It binds specifically to certain structures found in various sugars ...
reporter enzyme conjugate, which binds only glucose or mannose.
Classical qualitative detection reactions
These reactions have only historical significance:Fehling test
The Fehling test is a classic method for the detection of aldoses.H. Fehling: ''Quantitative Bestimmung des Zuckers im Harn''. In: '' Archiv für physiologische Heilkunde'' (1848), volume 7, p. 64–73 (in German). Due to mutarotation, glucose is always present to a small extent as an open-chain aldehyde. By adding the Fehling reagents (Fehling (I) solution and Fehling (II) solution), the aldehyde group is oxidized to a carboxylic acid, while the Cu2+ tartrate complex is reduced to Cu+ and forms a brick red precipitate (Cu2O).Tollens test
In the Tollens test, after addition of ammoniacal AgNO3 to the sample solution, glucose reduces Ag+ to elemental silver.Barfoed test
In Barfoed's test, a solution of dissolved copper acetate,sodium acetate
Sodium acetate, CH3COONa, also abbreviated Na O Ac, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Applications
Biotechnological
Sodium acetate is used as the carbon source for culturing bacteria ...
and acetic acid is added to the solution of the sugar to be tested and subsequently heated in a water bath for a few minutes. Glucose and other monosaccharides rapidly produce a reddish color and reddish brown copper(I) oxide
Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper, the other being or copper(II) oxide or cupric oxide (CuO). This red-coloured solid is a component of some antifoulin ...
(Cu2O).
Nylander's test
As a reducing sugar, glucose reacts in the Nylander's test.Other tests
Upon heating a dilutepotassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exp ...
solution with glucose to 100 °C, a strong reddish browning and a caramel-like odor develops.Georg Schwedt: ''Zuckersüße Chemie''. John Wiley & Sons, 2012, , p. 102 (in German). Concentrated sulfuric acid dissolves dry glucose without blackening at room temperature forming sugar sulfuric acid. In a yeast solution, alcoholic fermentation produces carbon dioxide in the ratio of 2.0454 molecules of glucose to one molecule of CO2. Glucose forms a black mass with stannous chloride
Tin(II) chloride, also known as stannous chloride, is a white crystalline solid with the formula . It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot. SnCl2 is widely used as a reducing agent (in acid ...
. In an ammoniacal silver solution, glucose (as well as lactose and dextrin) leads to the deposition of silver. In an ammoniacal lead acetate Lead acetate can refer to:
* Lead subacetate (Basic lead acetate), Pb3(OH)4(CH3COO)2
* Lead(IV) acetate
Lead(IV) acetate or lead tetraacetate is an organometallic compound with chemical formula . It is a colorless solid that is soluble in nonpo ...
solution, white lead glycoside is formed in the presence of glucose, which becomes less soluble on cooking and turns brown. In an ammoniacal copper solution, yellow copper oxide Copper oxide is a compound from the two elements copper and oxygen.
Copper oxide may refer to:
* Copper(I) oxide (cuprous oxide, Cu2O)
* Copper(II) oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black so ...
hydrate is formed with glucose at room temperature, while red copper oxide is formed during boiling (same with dextrin, except for with an ammoniacal copper acetate solution). With Hager's reagent, glucose forms mercury oxide during boiling. An alkaline bismuth
Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs ...
solution is used to precipitate elemental, black-brown bismuth with glucose. Glucose boiled in an ammonium molybdate Ammonium molybdate can refer to:
*Ammonium orthomolybdate, (NH4)2MoO4
*Ammonium heptamolybdate, (NH4)6Mo7O24, usually encountered as the tetrahydrate
*Ammonium phosphomolybdate, (NH4)3PMo12O40
*Ammonium tetrathiomolybdate, (NH4)2MoS4 this chemical i ...
solution turns the solution blue. A solution with indigo carmine
Indigo carmine, or 5,5′-indigodisulfonic acid sodium salt, is an organic salt derived from indigo by aromatic sulfonation, which renders the compound soluble in water. It is approved for use as a food colorant in the U.S and E.U. to produce ...
and sodium carbonate destains when boiled with glucose.
Instrumental quantification
Refractometry and polarimetry
In concentrated solutions of glucose with a low proportion of other carbohydrates, its concentration can be determined with a polarimeter. For sugar mixtures, the concentration can be determined with arefractometer
A refractometer is a laboratory or field device for the measurement of an index of refraction ( refractometry). The index of refraction is calculated from the observed refraction angle using Snell's law. For mixtures, the index of refraction the ...
, for example in the Oechsle determination in the course of the production of wine.
Photometric enzymatic methods in solution
The enzyme glucose oxidase (GOx) converts glucose into gluconic acid and hydrogen peroxide while consuming oxygen. Another enzyme, peroxidase, catalyzes a chromogenic reaction (Trinder reaction) of phenol with 4-aminoantipyrine to a purple dye.Photometric test-strip method
The test-strip method employs the above-mentioned enzymatic conversion of glucose to gluconic acid to form hydrogen peroxide. The reagents are immobilised on a polymer matrix, the so-called test strip, which assumes a more or less intense color. This can be measured reflectometrically at 510 nm with the aid of an LED-based handheld photometer. This allows routine blood sugar determination by nonscientists. In addition to the reaction of phenol with 4-aminoantipyrine, new chromogenic reactions have been developed that allow photometry at higher wavelengths (550 nm, 750 nm).Amperometric glucose sensor
The electroanalysis of glucose is also based on the enzymatic reaction mentioned above. The produced hydrogen peroxide can be amperometrically quantified by anodic oxidation at a potential of 600 mV. The GOx is immobilized on the electrode surface or in a membrane placed close to the electrode. Precious metals such as platinum or gold are used in electrodes, as well as carbon nanotube electrodes, which e.g. are doped with boron. Cu–CuO nanowires are also used as enzyme-free amperometric electrodes, reaching a detection limit of 50 μmol/L. A particularly promising method is the so-called "enzyme wiring", where the electron flowing during the oxidation is transferred via a molecular wire directly from the enzyme to the electrode.Other sensory methods
There are a variety of other chemical sensors for measuring glucose. Given the importance of glucose analysis in the life sciences, numerous optical probes have also been developed for saccharides based on the use of boronic acids, which are particularly useful for intracellular sensory applications where other (optical) methods are not or only conditionally usable. In addition to the organic boronic acid derivatives, which often bind highly specifically to the 1,2-diol groups of sugars, there are also other probe concepts classified by functional mechanisms which use selective glucose-binding proteins (e.g. concanavalin A) as a receptor. Furthermore, methods were developed which indirectly detect the glucose concentration via the concentration of metabolized products, e.g. by the consumption of oxygen using fluorescence-optical sensors. Finally, there are enzyme-based concepts that use the intrinsic absorbance or fluorescence of (fluorescence-labeled) enzymes as reporters.Copper iodometry
Glucose can be quantified by copper iodometry.Chromatographic methods
In particular, for the analysis of complex mixtures containing glucose, e.g. in honey, chromatographic methods such as high performance liquid chromatography and gas chromatography are often used in combination with mass spectrometry. Taking into account the isotope ratios, it is also possible to reliably detect honey adulteration by added sugars with these methods. Derivatization using silylation reagents is commonly used. Also, the proportions of di- and trisaccharides can be quantified.In vivo analysis
Glucose uptake in cells of organisms is measured with 2-deoxy-D-glucose orfluorodeoxyglucose
18F.html" ;"title="sup>18F">sup>18Fluorodeoxyglucose ( INN), or fluorodeoxyglucose F 18 (USAN and USP), also commonly called fluorodeoxyglucose and abbreviated 18F.html" ;"title="sup>18F">sup>18FDG, 2- 18F.html" ;"title="sup>18F">sup>18FDG or ...
.Donard Dwyer: ''Glucose Metabolism in the Brain.'' Academic Press, 2002, , p. XIII. (18F)fluorodeoxyglucose is used as a tracer in positron emission tomography in oncology and neurology,Gesellschaft Deutscher Chemiker
The German Chemical Society (German: ', GDCh) is a learned society and professional association founded in 1949 to represent the interests of German chemists in local, national and international contexts. GDCh "brings together people working in che ...
wayback=20100331071121 ''Anlagen zum Positionspapier der Fachgruppe Nuklearchemie''
, February 2000. where it is by far the most commonly used diagnostic agent.
References
External links
* * {{portal bar, Chemistry, Medicine Chemical pathology Nutrition World Health Organization essential medicines Pyranoses Glycolysis