Graphene Nanoribbon Field-effect Transistor on:
[Wikipedia]
[Google]
[Amazon]
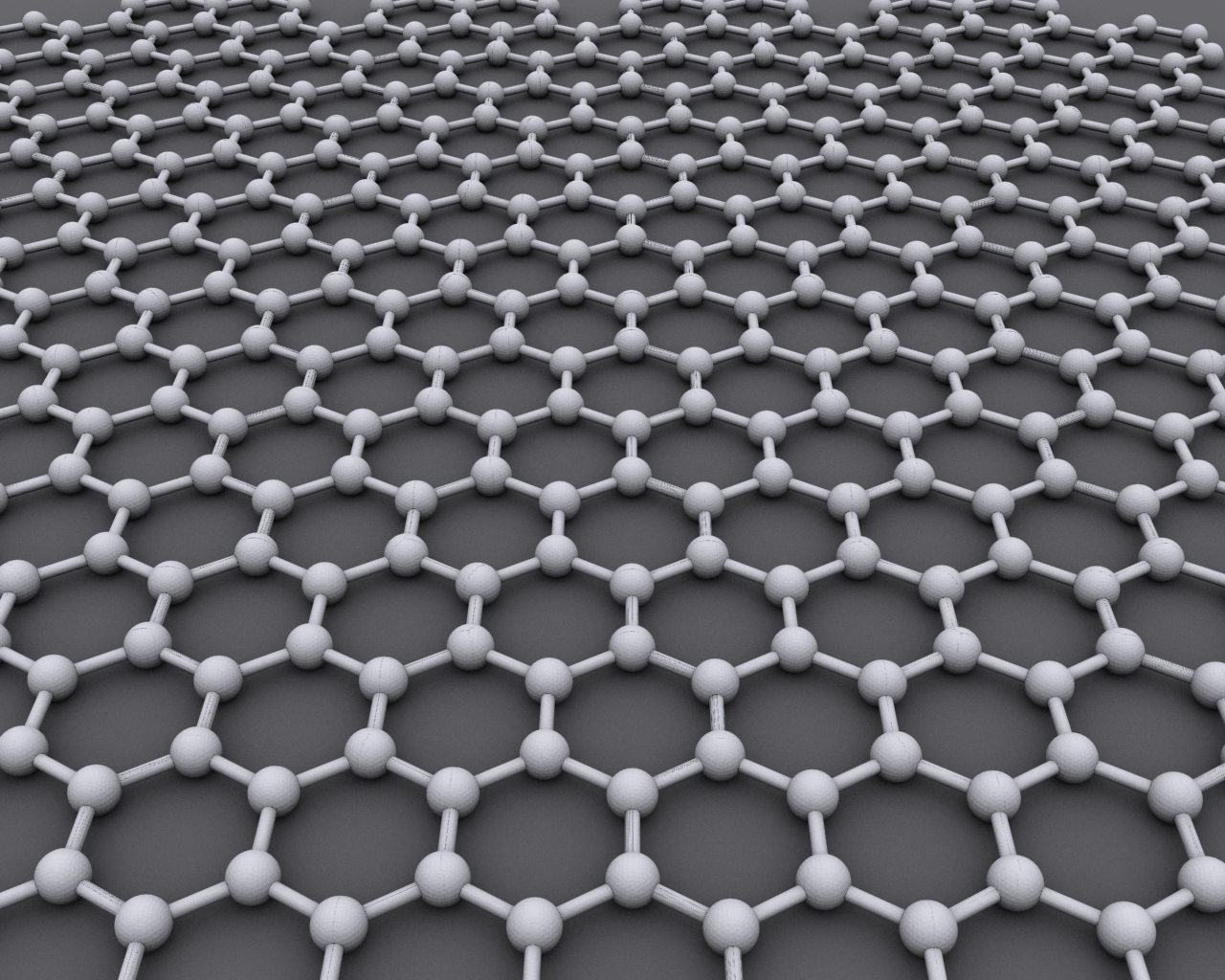 Graphene () is an
Graphene () is an
"Carbon nanostructures for electromagnetic shielding applications", Mohammed Arif Poothanari, Sabu Thomas, et al., ''Industrial Applications of Nanomaterials'', 2019. "Carbon nanostructures include various low-dimensional allotropes of carbon including carbon black (CB), carbon fiber, carbon nanotubes (CNTs), fullerene, and graphene." The name is derived from "graphite" and the suffix
 Graphene () is an
Graphene () is an allotrope of carbon
Carbon is capable of forming many allotropes (structurally different forms of the same element) due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and rese ...
consisting of a single layer of atoms arranged in a hexagonal lattice
The hexagonal lattice or triangular lattice is one of the five two-dimensional Bravais lattice types. The symmetry category of the lattice is wallpaper group p6m. The primitive translation vectors of the hexagonal lattice form an angle of 120° ...
nanostructure
A nanostructure is a structure of intermediate size between microscopic and molecular structures. Nanostructural detail is microstructure at nanoscale.
In describing nanostructures, it is necessary to differentiate between the number of di ...
."Carbon nanostructures for electromagnetic shielding applications", Mohammed Arif Poothanari, Sabu Thomas, et al., ''Industrial Applications of Nanomaterials'', 2019. "Carbon nanostructures include various low-dimensional allotropes of carbon including carbon black (CB), carbon fiber, carbon nanotubes (CNTs), fullerene, and graphene." The name is derived from "graphite" and the suffix
-ene
The suffix -ene is used in organic chemistry to form names of organic compounds where the -C=C- group has been attributed the highest priority according to the rules of organic nomenclature. Sometimes a number between hyphens is inserted before ...
, reflecting the fact that the graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on la ...
allotrope of carbon contains numerous double bonds.
Each atom in a graphene sheet is connected to its three neare