Gilman Reagent on:
[Wikipedia]
[Google]
[Amazon]
A Gilman reagent is a diorganocopper compound with the formula Li uR2 where R is an
 These reagents are useful because, unlike related
These reagents are useful because, unlike related

 If the Li+ ions is complexed with the crown ether 12-crown-4, the resulting diorganylcuprate anions adopt a linear coordination geometry at copper.
If the Li+ ions is complexed with the crown ether 12-crown-4, the resulting diorganylcuprate anions adopt a linear coordination geometry at copper.

 For the 'higher order cyanocuprate' Li2CuCN(CH3)2, Lipshutz and coworkers have claimed that the cyanide ligand is coordinated to Li and π-bound to Cu. However, the existence of 'mixed higher order organocuprates' has been disputed by Bertz and coworkers, who rejoined that the cyano ligand is actually bound solely to the lithium atom, and that such a structure could still explain the enhanced reactivity of cuprate prepared from CuCN. To date, no crystallographic evidence for the existence of 'mixed higher order cuprates' ( 2CuXsup>2–, X ≠ R) has been obtained. On the other hand, a homoleptic higher order cuprate in the form of a h3Cusup>2– moiety has been observed in Li3Cu2Ph5(SMe2)4, prepared by Olmstead and Power.
For the 'higher order cyanocuprate' Li2CuCN(CH3)2, Lipshutz and coworkers have claimed that the cyanide ligand is coordinated to Li and π-bound to Cu. However, the existence of 'mixed higher order organocuprates' has been disputed by Bertz and coworkers, who rejoined that the cyano ligand is actually bound solely to the lithium atom, and that such a structure could still explain the enhanced reactivity of cuprate prepared from CuCN. To date, no crystallographic evidence for the existence of 'mixed higher order cuprates' ( 2CuXsup>2–, X ≠ R) has been obtained. On the other hand, a homoleptic higher order cuprate in the form of a h3Cusup>2– moiety has been observed in Li3Cu2Ph5(SMe2)4, prepared by Olmstead and Power.
alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
. They are colorless solids.
Use in organic chemistry
 These reagents are useful because, unlike related
These reagents are useful because, unlike related Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
s and organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
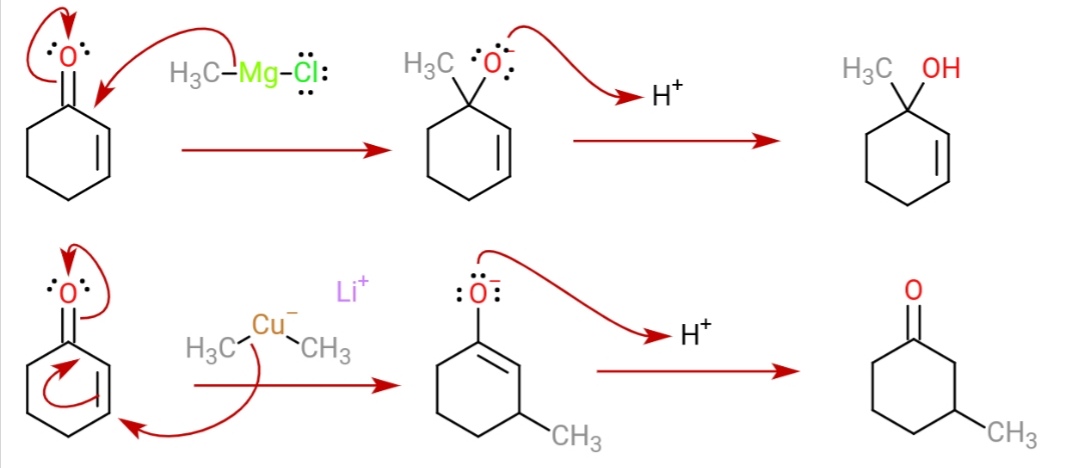
s, they react with organic halides to replace the halide group with an R group (the Corey–House reaction). Such displacement reactions allow for the synthesis of complex products from simple building blocks. Lewis acids can be used to modify the reagent.
History
These reagents were discovered by Henry Gilman and coworkers. Lithium dimethylcopper (CH3)2CuLi can be prepared by adding copper(I) iodide tomethyllithium
Methyllithium is the simplest organolithium reagent, with the empirical formula LiCH3. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used i ...
in tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
at −78 °C. In the reaction depicted below, the Gilman reagent is a methylating reagent reacting with an alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
in a conjugate addition, and the ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
group forms a cyclic enone.
:
Structure
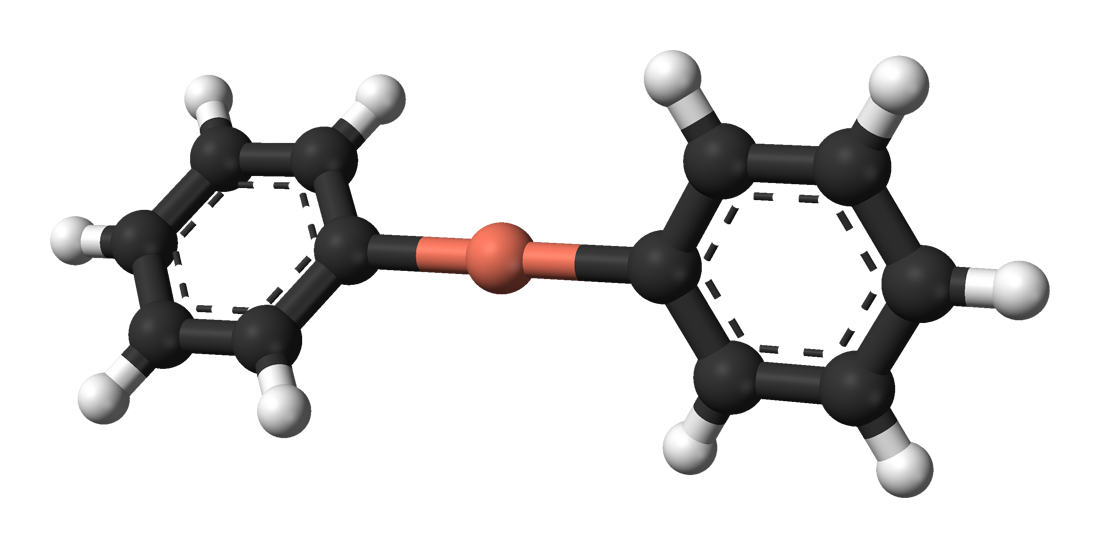
Lithium dimethylcuprate exists as a dimer indiethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
forming an 8-membered ring. Similarly, lithium diphenylcuprate crystallizes as a dimeric etherate, .




Mixed cuprates
More useful generally than the Gilman reagents are the so-called mixed cuprates with the formula CuXsup>− and 2CuXsup>2− (see above for the controversy over existence of the latter). Such compounds are often prepared by the addition of the organolithium reagent to copper(I) halides and cyanide. These mixed cuprates are more stable and more readily purified.Steven H. Bertz, Edward H. Fairchild, Karl Dieter, "Copper(I) Cyanide" in Encyclopedia of Reagents for Organic Synthesis 2005, John Wiley & Sons. One problem addressed by mixed cuprates is the economical use of the alkyl group. Thus, in some applications, the mixed cuprate with the formula is prepared by combining thienyllithium and cuprous cyanide followed by the organic group to be transferred. In this higher order mixed cuprate, both the cyanide and thienyl groups do not transfer, only the R group does. Bruce H. Lipshutz, Robert Moretti, Robert Crow "Mixed Higher-order Cyanocuprate-induced Epoxide Openings: 1-Benzyloxy-4-penten-2-ol" Org. Synth. 1990, volume 69, pp. 80.See also
* Cuprate (chemistry)References
{{Lithium compounds Organolithium compounds Organocopper compounds Reagents for organic chemistry