Gas Thermometer on:
[Wikipedia]
[Google]
[Amazon]
 A gas thermometer is a
A gas thermometer is a
 :
The constant volume gas thermometer plays a crucial role in understanding how
:
The constant volume gas thermometer plays a crucial role in understanding how
 A gas thermometer is a
A gas thermometer is a thermometer
A thermometer is a device that measures temperature (the hotness or coldness of an object) or temperature gradient (the rates of change of temperature in space). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb ...
that measures the temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
of a gas by variation in the volume or pressure of the gas.
Constant-pressure thermometer
According toCharles's law
Charles's law (also known as the law of volumes) is an experimental gas law that describes how gases tend to expand when heated. A modern statement of Charles's law is:
When the pressure on a sample of a dry gas is held constant, the Kelvin ...
, the volume of gas is directly proportional to the temperature of that gas, when its pressure and mass are kept constant; that is,
:
In other words,
:
where is the volume, is the thermodynamic temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
and is the constant for the system. The constant is not a fixed constant across all systems and therefore needs to be found experimentally for a given system through testing with known temperature values.
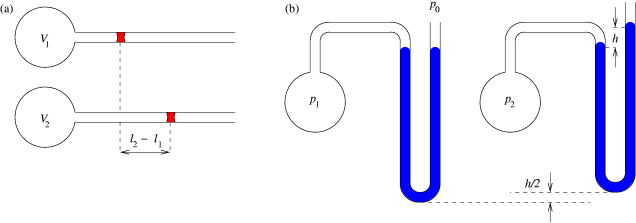
This works on the same principle as mercury thermometers. If a change in volume can be measured with one standard known temperature (such as the melting point of water), the second unknown temperature can be determined.
Constant-volume thermometer and absolute zero
The constant-volume gas thermometer works on the basis of the pressure-temperature law: when the volume of a gas is kept constant, its pressure is directly proportional its temperature. : That is, :
The constant volume gas thermometer plays a crucial role in understanding how
:
The constant volume gas thermometer plays a crucial role in understanding how absolute zero
Absolute zero is the lowest possible temperature, a state at which a system's internal energy, and in ideal cases entropy, reach their minimum values. The absolute zero is defined as 0 K on the Kelvin scale, equivalent to −273.15 ° ...
could be discovered long before the advent of cryogenics
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th International Institute of Refrigeration's (IIR) International Congress of Refrigeration (held in Washington, DC in 1971) endorsed a universa ...
.
Consider a graph of pressure versus temperature made around standard conditions (well above absolute zero) for three different samples of any ideal gas ''(a, b, c)''. To the extent that the gas is ideal, the pressure depends linearly on temperature, and the extrapolation to zero pressure occurs at absolute zero. Note that data could have been collected with three different amounts of the same gas, which would have rendered this experiment easy to do in the eighteenth century.
History
See also
*Thermodynamic instruments
A thermodynamic instrument is any device for the measurement of thermodynamic systems. In order for a thermodynamic parameter or physical quantity to be truly defined, a technique for its measurement must be specified. For example, the ultimate def ...
* Boyle's law
Boyle's law, also referred to as the Boyle–Mariotte law or Mariotte's law (especially in France), is an empirical gas laws, gas law that describes the relationship between pressure and volume of a confined gas. Boyle's law has been stated as:
...
* Combined gas law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first sta ...
* Gay-Lussac's law
Gay-Lussac's law usually refers to Joseph-Louis Gay-Lussac's law of combining volumes of gases, discovered in 1808 and published in 1809. However, it sometimes refers to the proportionality of the volume of a gas to its Thermodynamic temperature ...
* Avogadro's law
Avogadro's law (sometimes referred to as Avogadro's hypothesis or Avogadro's principle) or Avogadro-Ampère's hypothesis is an experimental gas law relating the volume of a gas to the amount of substance of gas present. The law is a specific cas ...
* Ideal gas law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stat ...
References