first generation solar cell on:
[Wikipedia]
[Google]
[Amazon]
 Crystalline silicon or (c-Si) Is the crystalline forms of silicon, either
Crystalline silicon or (c-Si) Is the crystalline forms of silicon, either
 In 2013, conventional crystalline silicon technology dominated worldwide PV production, with multi-Si leading the market ahead of mono-Si, accounting for 54% and 36%, respectively. For the last ten years, worldwide market-share of thin-film technologies stagnated below 18% and currently stand at 9%. In the thin-film market, CdTe leads with an annual production of 2 GW p or 5%, followed by a-Si and CIGS, both around 2%.
Alltime deployed PV capacity of 139 gigawatts ( cumulative as of 2013) splits up into 121 GW crystalline silicon (87%) and 18 GW thin-film (13%) technology.
In 2013, conventional crystalline silicon technology dominated worldwide PV production, with multi-Si leading the market ahead of mono-Si, accounting for 54% and 36%, respectively. For the last ten years, worldwide market-share of thin-film technologies stagnated below 18% and currently stand at 9%. In the thin-film market, CdTe leads with an annual production of 2 GW p or 5%, followed by a-Si and CIGS, both around 2%.
Alltime deployed PV capacity of 139 gigawatts ( cumulative as of 2013) splits up into 121 GW crystalline silicon (87%) and 18 GW thin-film (13%) technology.
 The
The
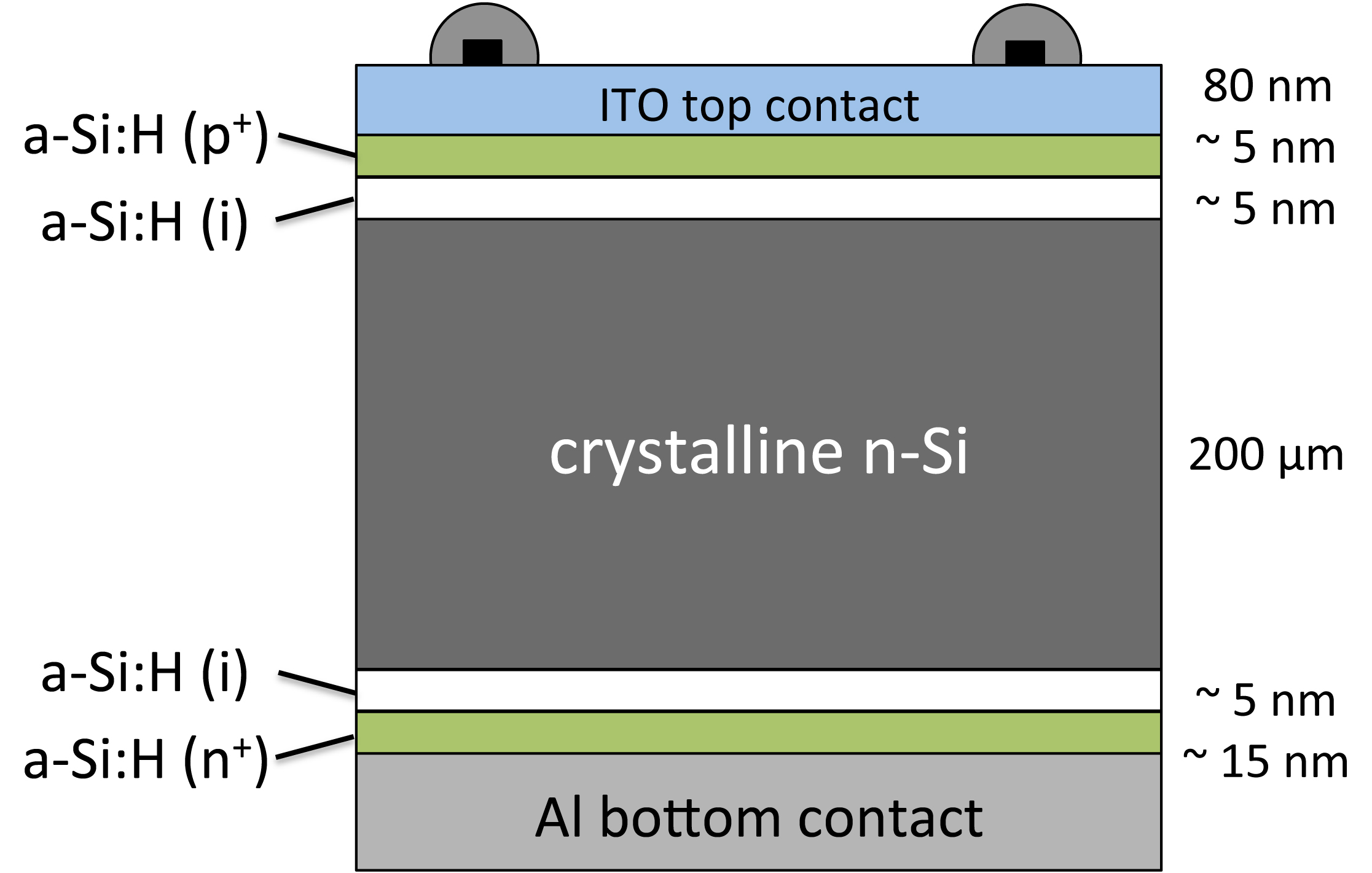 A HIT solar cell is composed of a mono thin crystalline silicon wafer surrounded by ultra-thin
A HIT solar cell is composed of a mono thin crystalline silicon wafer surrounded by ultra-thin
 Monocrystalline silicon (mono c-Si) is a form in which the crystal structure is homogeneous throughout the material; the orientation, lattice parameter, and electronic properties are constant throughout the material.. Dopant atoms such as phosphorus and boron are often incorporated into the film to make the silicon n-type or p-type respectively. Monocrystalline silicon is fabricated in the form of silicon wafers, usually by the Czochralski Growth method, and can be quite expensive depending on the radial size of the desired single crystal wafer (around $200 for a 300 mm Si wafer). This monocrystalline material, while useful, is one of the chief expenses associated with producing photovoltaics where approximately 40% of the final price of the product is attributable to the cost of the starting silicon wafer used in cell fabrication.
Monocrystalline silicon (mono c-Si) is a form in which the crystal structure is homogeneous throughout the material; the orientation, lattice parameter, and electronic properties are constant throughout the material.. Dopant atoms such as phosphorus and boron are often incorporated into the film to make the silicon n-type or p-type respectively. Monocrystalline silicon is fabricated in the form of silicon wafers, usually by the Czochralski Growth method, and can be quite expensive depending on the radial size of the desired single crystal wafer (around $200 for a 300 mm Si wafer). This monocrystalline material, while useful, is one of the chief expenses associated with producing photovoltaics where approximately 40% of the final price of the product is attributable to the cost of the starting silicon wafer used in cell fabrication.
 Crystalline silicon or (c-Si) Is the crystalline forms of silicon, either
Crystalline silicon or (c-Si) Is the crystalline forms of silicon, either polycrystalline silicon
Polycrystalline silicon, or multicrystalline silicon, also called polysilicon, poly-Si, or mc-Si, is a high purity, polycrystalline form of silicon, used as a raw material by the solar photovoltaic and electronics industry.
Polysilicon is produce ...
(poly-Si, consisting of small crystals), or monocrystalline silicon (mono-Si, a continuous crystal). Crystalline silicon is the dominant semiconducting material
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way. ...
used in photovoltaic
Photovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect, a phenomenon studied in physics, photochemistry, and electrochemistry. The photovoltaic effect is commercially u ...
technology for the production of solar cells. These cells are assembled into solar panels as part of a photovoltaic system
A photovoltaic system, also PV system or solar power system, is an electric power system designed to supply usable solar power by means of photovoltaics. It consists of an arrangement of several components, including solar panels to absorb and co ...
to generate solar power
Solar power is the conversion of energy from sunlight into electricity, either directly using photovoltaics (PV) or indirectly using concentrated solar power. Photovoltaic cells convert light into an electric current using the photovoltaic e ...
from sunlight.
In electronics, crystalline silicon is typically the monocrystalline form of silicon, and is used for producing microchips
An integrated circuit or monolithic integrated circuit (also referred to as an IC, a chip, or a microchip) is a set of electronic circuits on one small flat piece (or "chip") of semiconductor material, usually silicon. Transistor count, Large ...
. This silicon contains much lower impurity levels than those required for solar cells. Production of semiconductor grade silicon involves a chemical purification to produce Hyper-pure Polysilicon, followed by a recrystallization process to grow monocrystalline silicon. The cylindrical boules
''Boules'' () is a collective name for a wide range of games similar to bowls and bocce (In French: jeu or jeux, in Croatian: boćanje and in Italian: gioco or giochi) in which the objective is to throw or roll heavy balls (called in France, ...
are then cut into wafers
A wafer is a crisp, often sweet, very thin, flat, light and dry biscuit, often used to decorate ice cream, and also used as a garnish on some sweet dishes. Wafers can also be made into cookies with cream flavoring sandwiched between them. They ...
for further processing.
Solar cells made of crystalline silicon are often called ''conventional'', ''traditional'', or ''first generation'' solar cells, as they were developed in the 1950s and remained the most common type up to the present time. Because they are produced from 160–190 μm
The micrometre ( international spelling as used by the International Bureau of Weights and Measures; SI symbol: μm) or micrometer ( American spelling), also commonly known as a micron, is a unit of length in the International System of Uni ...
thick solar wafer
In electronics, a wafer (also called a slice or substrate) is a thin slice of semiconductor, such as a crystalline silicon (c-Si), used for the fabrication of integrated circuits and, in photovoltaics, to manufacture solar cells. The wafer serv ...
s—slices from bulks of solar grade silicon—they are sometimes called ''wafer-based'' solar cells.
Solar cells made from c-Si are single-junction cell
A unijunction transistor (UJT) is a three-lead electronic semiconductor device with only one junction that acts exclusively as an electrically controlled switch.
The UJT is not used as a linear amplifier. It is used in free-running oscillators, ...
s and are generally more efficient than their rival technologies, which are the second-generation thin-film solar cells, the most important being CdTe
Cadmium telluride (CdTe) is a stable crystalline compound formed from cadmium and tellurium. It is mainly used as the semiconducting material in cadmium telluride photovoltaics and an infrared optical window. It is usually sandwiched with cadm ...
, CIGS, and amorphous silicon
Amorphous silicon (a-Si) is the non-crystalline form of silicon used for solar cells and thin-film transistors in LCDs.
Used as semiconductor material for a-Si solar cells, or thin-film silicon solar cells, it is deposited in thin films onto ...
(a-Si). Amorphous silicon is an allotropic
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
variant of silicon, and amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek ''a'' ("wit ...
means "without shape" to describe its non-crystalline form.
Overview
Classification
The allotropic forms of silicon range from a single crystalline structure to a completely unordered amorphous structure with several intermediate varieties. In addition, each of these different forms can possess several names and even more abbreviations, and often cause confusion to non-experts, especially as some materials and their application as a PV technology are of minor significance, while other materials are of outstanding importance.PV industry
The photovoltaic industry, however, groups them into two distinct categories: * crystalline silicon (c-Si), used in traditional, conventional,wafer
A wafer is a crisp, often sweet, very thin, flat, light and dry biscuit, often used to decorate ice cream, and also used as a garnish on some sweet dishes. Wafers can also be made into cookies with cream flavoring sandwiched between them. They ...
-based solar cells:
** monocrystalline silicon (mono-Si)
** polycrystalline silicon
Polycrystalline silicon, or multicrystalline silicon, also called polysilicon, poly-Si, or mc-Si, is a high purity, polycrystalline form of silicon, used as a raw material by the solar photovoltaic and electronics industry.
Polysilicon is produce ...
(multi-Si)
** ribbon silicon (ribbon-Si), has currently no market
* not classified as crystalline silicon, used in thin-film and other solar-cell technologies:
** amorphous silicon
Amorphous silicon (a-Si) is the non-crystalline form of silicon used for solar cells and thin-film transistors in LCDs.
Used as semiconductor material for a-Si solar cells, or thin-film silicon solar cells, it is deposited in thin films onto ...
(a-Si)
** nanocrystalline silicon
Nanocrystalline silicon (nc-Si), sometimes also known as microcrystalline silicon (μc-Si), is a form of porous silicon. It is an allotropic form of silicon with paracrystalline structure—is similar to amorphous silicon (a-Si), in that it has ...
(nc-Si)
** protocrystalline silicon (pc-Si)
** other non-silicon materials, such as CdTe
Cadmium telluride (CdTe) is a stable crystalline compound formed from cadmium and tellurium. It is mainly used as the semiconducting material in cadmium telluride photovoltaics and an infrared optical window. It is usually sandwiched with cadm ...
, CIGS
** emerging photovoltaics
Third-generation photovoltaic cells are solar cells that are potentially able to overcome the Shockley–Queisser limit of 31–41% power efficiency for single bandgap solar cells. This includes a range of alternatives to cells made of semiconducti ...
** multi-junction solar cells (MJ) commonly used for solar panels on spacecraft
Spacecraft operating in the inner Solar System usually rely on the use of power electronics-managed photovoltaic solar panels to derive electricity from sunlight. Outside the orbit of Jupiter, solar radiation is too weak to produce sufficient po ...
for space-based solar power. They are also used in concentrator photovoltaics
Concentrator photovoltaics (CPV) (also known as concentration photovoltaics) is a photovoltaic technology that generates electricity from sunlight. Unlike conventional photovoltaic systems, it uses lenses or curved mirrors to focus sunlight onto ...
(CPV, HCPV), an emerging technology best suited for locations that receive much sunlight.
Generations
Alternatively, different types of solar cells and/or their semiconducting materials can be classified by generations: * First generation solar cells are made of crystalline silicon, also called, conventional, traditional, wafer-based solar cells and include monocrystalline (mono-Si) and polycrystalline (multi-Si) semiconducting materials. * Second generation solar cells or panels are based on thin-film technology and are of commercially significant importance. These include CdTe, CIGS and amorphous silicon. * Third generation solar cells are often labeled as ''emerging technologies'' with little or no market significance and include a large range of substances, mostly organic, often using organometallic compounds. Arguably, multi-junction photovoltaic cells can be classified to neither of these generations. A typical triple junction semiconductor is made ofInGaP
Indium gallium phosphide (InGaP), also called gallium indium phosphide (GaInP), is a semiconductor composed of indium, gallium and phosphorus. It is used in high-power and high-frequency electronics because of its superior electron velocity with ...
/ (In)GaAs/ Ge.
Comparison of technical specifications
Market share
Efficiency
 The
The conversion efficiency
Energy conversion efficiency (''η'') is the ratio between the useful output of an energy conversion machine and the input, in energy terms. The input, as well as the useful output may be chemical, electric power, mechanical work, light (radi ...
of PV devices describes the energy-ratio of the outgoing electrical power compared to the incoming radiated light. A single solar cells has generally a better, or higher efficiency than an entire solar module. Also lab efficiency is always significantly ahead of commercially available products in the market.
; Lab cells
In 2013, record Lab cell efficiency was highest for crystalline silicon. However, multi-silicon is followed closely by cadmium telluride and copper indium gallium selenide solar cells.
# 25.6% ------- mono-Si cell
# 20.4% -------- multi-Si cell
# 21.7% ----------- CIGS cell
# 21.5% ----------- CdTe cell
Both-sides-contacted silicon solar cells as of 2021: 26% and possibly above.
; Modules
The average commercial crystalline silicon module increased its efficiency from about 12% to 16% over the last ten years. In the same period CdTe-modules improved their efficiency from 9 to 16%.
The modules performing best under lab conditions in 2014 were made of monocrystalline silicon.
They were 7% above the efficiency of commercially produced modules (23% over 16%) which indicated that the conventional silicon technology still had potential to improve and therefore maintain its leading position.
Energy costs of manufacture
Crystalline silicon has a high cost in energy because silicon is produced by the reduction of high-grade quartz sand in an electric furnace. The electricity generated for this process may producegreenhouse gas emissions
Greenhouse gas emissions from human activities strengthen the greenhouse effect, contributing to climate change. Most is carbon dioxide from burning fossil fuels: coal, oil, and natural gas. The largest emitters include coal in China and lar ...
. This coke-fired smelting
Smelting is a process of applying heat to ore, to extract a base metal. It is a form of extractive metallurgy. It is used to extract many metals from their ores, including silver, iron, copper, and other base metals. Smelting uses heat and a c ...
process occurs at high temperatures of more than 1,000°C and is very energy intensive, using about 11 kilowatt-hours (kWh) per kilogram of silicon.
The energy requirements of this process per unit of silicon metal produced may be relatively inelastic. But major energy cost reductions per (photovoltaic) product have been made as silicon cells have become more efficient at converting sunlight, larger silicon metal ingots are cut with less waste into thinner wafers, silicon waste from manufacture is recycled, and material costs have reduced.
Toxicity
With the exception ofamorphous silicon
Amorphous silicon (a-Si) is the non-crystalline form of silicon used for solar cells and thin-film transistors in LCDs.
Used as semiconductor material for a-Si solar cells, or thin-film silicon solar cells, it is deposited in thin films onto ...
, most commercially established PV technologies use toxic heavy metals. CIGS often uses a CdS buffer layer, and the semiconductor material of CdTe
Cadmium telluride (CdTe) is a stable crystalline compound formed from cadmium and tellurium. It is mainly used as the semiconducting material in cadmium telluride photovoltaics and an infrared optical window. It is usually sandwiched with cadm ...
-technology itself contains the toxic cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of ...
(Cd). In the case of crystalline silicon modules, the solder material that joins together the copper strings of the cells, it contains about 36% of lead (Pb). Moreover, the paste used for screen printing front and back contacts contains traces of Pb and sometimes Cd as well. It is estimated that about 1,000 metric tonnes of Pb have been used for 100 gigawatts of c-Si solar modules. However, there is no fundamental need for lead in the solder alloy.
Cell technologies
PERC solar cell
Passivated emitter rear contact (PERC) solar cells consist of the addition of an extra layer to the rear-side of a solar cell. This dielectric passive layer acts to reflect unabsorbed light back to the solar cell for a second absorption attempt increasing the solar cell efficiency. A PERC is created through an additional film deposition and etching process. Etching can be done either by chemical or laser processing.HIT solar cell
 A HIT solar cell is composed of a mono thin crystalline silicon wafer surrounded by ultra-thin
A HIT solar cell is composed of a mono thin crystalline silicon wafer surrounded by ultra-thin amorphous silicon
Amorphous silicon (a-Si) is the non-crystalline form of silicon used for solar cells and thin-film transistors in LCDs.
Used as semiconductor material for a-Si solar cells, or thin-film silicon solar cells, it is deposited in thin films onto ...
layers. The acronym HIT stands for " heterojunction with intrinsic thin layer". HIT cells are produced by the Japanese multinational electronics corporation Panasonic (see also ). Panasonic and several other groups have reported several advantages of the HIT design over its traditional c-Si counterpart:
1. An intrinsic a-Si layer can act as an effective surface passivation layer for c-Si
wafer.
2. The p+/n+ doped a-Si functions as an effective emitter/BSF for the cell.
3. The a-Si layers are deposited at much lower temperature, compared to the processing temperatures for traditional diffused c-Si technology.
4. The HIT cell has a lower temperature coefficient compared to c-Si cell technology.
Owing to all these advantages, this new hetero-junction solar cell is a considered to be a promising low cost alternative to traditional c-Si based solar cells.
Fabrication of HIT cells
The details of the fabrication sequence vary from group to group. Typically in good quality, CZ/FZ grown c-Si wafer (with ~1ms lifetimes) are used as the absorber layer of HIT cells. Using alkaline etchants, such as, NaOH or (CH3)4NOH the (100) surface of the wafer is textured to form the pyramids of 5-10μm height. Next, the wafer is cleaned using peroxide and HF solutions. This is followed by deposition of intrinsic a-Si passivation layer, typically through PECVD or Hot-wire CVD. The silane (SiH4) gas diluted with H2 is used as a precursor. The deposition temperature and pressure is maintained at 200o C and 0.1-1 Torr. Precise control over this step is essential to avoid the formation of defective epitaxial Si. Cycles of deposition and annealing and H2 plasma treatment are shown to have provided excellent surface passivation. Diborane or Trimethylboron gas mixed with SiH4 is used to deposit p-type a-Si layer, while, Phosphine gas mixed with SiH4 is used to deposit n-type a-Si layer. Direct deposition of doped a-Si layers on c-Si wafer is shown to have very poor passivation properties. This is most likely due to dopant induced defect generation in a-Si layers. Sputtered Indium Tin Oxide (ITO) is commonly used as a transparent conductive oxide (TCO) layer on top of the front and back a-Si layer in bi-facial design, as a-Si has high lateral resistance. It is generally deposited on the back side as well fully metallized cell to avoid diffusion of back metal and also for impedance matching for the reflected light. The silver/aluminum grid of 50-100μm thick is deposited through stencil printing for the front contact and back contact for bi-facial design. The detailed description of the fabrication process can be found in.Opto-electrical modeling and characterization of HIT cells
The literature discusses several studies to interpret carrier transport bottlenecks in these cells. Traditional light and dark I-V are extensively studied and are observed to have several non-trivial features, which cannot be explained using the traditional solar cell diode theory. This is because of the presence of hetero-junction between the intrinsic a-Si layer and c-Si wafer which introduces additional complexities to current flow. In addition, there has been significant efforts to characterize this solar cell using C-V, impedance spectroscopy, surface photo-voltage, suns-Voc to produce complementary information. Further, a number of design improvements, such as, the use of new emitters, bifacial configuration, interdigitated back contact (IBC) configuration bifacial-tandem configuration are actively being pursued.Mono-silicon
Polycrystalline silicon
Polycrystalline silicon
Polycrystalline silicon, or multicrystalline silicon, also called polysilicon, poly-Si, or mc-Si, is a high purity, polycrystalline form of silicon, used as a raw material by the solar photovoltaic and electronics industry.
Polysilicon is produce ...
is composed of many smaller silicon grains of varied crystallographic orientation, typically >1 mm in size. This material can be synthesized easily by allowing liquid silicon to cool using a seed crystal of the desired crystal structure. Additionally, other methods for forming smaller-grained polycrystalline silicon (poly-Si) exist such as high temperature chemical vapor deposition (CVD).
Not classified as Crystalline silicon
These allotropic forms of silicon are not classified as crystalline silicon. They belong to the group of thin-film solar cells.Amorphous silicon
Amorphous silicon (a-Si) has no long-range periodic order. The application of amorphous silicon to photovoltaics as a standalone material is somewhat limited by its inferior electronic properties.. When paired with microcrystalline silicon in tandem and triple-junction solar cells, however, higher efficiency can be attained than with single-junction solar cells. This tandem assembly of solar cells allows one to obtain a thin-film material with a bandgap of around 1.12 eV (the same as single-crystal silicon) compared to the bandgap of amorphous silicon of 1.7-1.8 eV bandgap. Tandem solar cells are then attractive since they can be fabricated with a bandgap similar to single-crystal silicon but with the ease of amorphous silicon.Nanocrystalline silicon
Nanocrystalline silicon
Nanocrystalline silicon (nc-Si), sometimes also known as microcrystalline silicon (μc-Si), is a form of porous silicon. It is an allotropic form of silicon with paracrystalline structure—is similar to amorphous silicon (a-Si), in that it has ...
(nc-Si), sometimes also known as ''microcrystalline silicon'' (μc-Si), is a form of porous silicon. It is an allotropic
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
form of silicon with paracrystalline
In materials science, paracrystalline materials are defined as having short- and medium-range ordering in their lattice (similar to the liquid crystal phases) but lacking crystal-like long-range ordering at least in one direction.
Origin and ...
structure—is similar to amorphous silicon
Amorphous silicon (a-Si) is the non-crystalline form of silicon used for solar cells and thin-film transistors in LCDs.
Used as semiconductor material for a-Si solar cells, or thin-film silicon solar cells, it is deposited in thin films onto ...
(a-Si), in that it has an amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek ''a'' ("wit ...
phase. Where they differ, however, is that nc-Si has small grains of crystalline silicon within the amorphous phase. This is in contrast to polycrystalline silicon
Polycrystalline silicon, or multicrystalline silicon, also called polysilicon, poly-Si, or mc-Si, is a high purity, polycrystalline form of silicon, used as a raw material by the solar photovoltaic and electronics industry.
Polysilicon is produce ...
(poly-Si) which consists solely of crystalline silicon grains, separated by grain boundaries. The difference comes solely from the grain size of the crystalline grains. Most materials with grains in the micrometre range are actually fine-grained polysilicon, so nanocrystalline silicon is a better term. The term Nanocrystalline silicon refers to a range of materials around the transition region from amorphous to microcrystalline phase in the silicon thin film.
Protocrystalline silicon
Protocrystalline silicon has a higher efficiency than amorphous silicon (a-Si) and it has also been shown to improve stability, but not eliminate it. A Protocrystalline phase is a distinct phase occurring duringcrystal growth
A crystal is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. Crystal growth is a major stage of a crystallization process, and consists of the ...
which evolves into a microcrystalline form.
Protocrystalline Si also has a relatively low absorption near the band gap owing to its more ordered crystalline structure. Thus, protocrystalline and amorphous silicon can be combined in a tandem solar cell where the top layer of thin protocrystalline silicon absorbs short-wavelength light whereas the longer wavelengths are absorbed by the underlying a-Si substrate.
Transformation of amorphous into crystalline silicon
Amorphous silicon can be transformed to crystalline silicon using well-understood and widely implemented high-temperature annealing processes. The typical method used in industry requires high-temperature compatible materials, such as special high temperature glass that is expensive to produce. However, there are many applications for which this is an inherently unattractive production method.Low temperature induced crystallization
Flexible solar cells have been a topic of interest for less conspicuous-integrated power generation than solar power farms. These modules may be placed in areas where traditional cells would not be feasible, such as wrapped around a telephone pole or cell phone tower. In this application a photovoltaic material may be applied to a flexible substrate, often a polymer. Such substrates cannot survive the high temperatures experienced during traditional annealing. Instead, novel methods of crystallizing the silicon without disturbing the underlying substrate have been studied extensively. Aluminum-induced crystallization (AIC) and local laser crystallization are common in the literature, however not extensively used in industry. In both of these methods, amorphous silicon is grown using traditional techniques such as plasma-enhanced chemical vapor deposition (PECVD). The crystallization methods diverge during post-deposition processing. In aluminum-induced crystallization, a thin layer of aluminum (50 nm or less) is deposited by physical vapor deposition onto the surface of the amorphous silicon. This stack of material is then annealed at a relatively low temperature between 140 °C and 200 °C in a vacuum. The aluminum that diffuses into the amorphous silicon is believed to weaken the hydrogen bonds present, allowing crystal nucleation and growth.. Experiments have shown that polycrystalline silicon with grains on the order of 0.2 – 0.3 μm can be produced at temperatures as low as 150 °C. The volume fraction of the film that is crystallized is dependent on the length of the annealing process. In aluminum-induced crystallization, a thin layer of aluminum (50 nm or less) is deposited by physical vapor deposition onto the surface of the amorphous silicon. This stack of material is then annealed at a relatively low temperature between 140°C and 200°C in a vacuum. The aluminum that diffuses into the amorphous silicon is believed to weaken the hydrogen bonds present, allowing crystal nucleation and growth.. Experiments have shown that polycrystalline silicon with grains on the order of 0.2 – 0.3 μm can be produced at temperatures as low as 150°C. The volume fraction of the film that is crystallized is dependent on the length of the annealing process. Aluminum-induced crystallization produces polycrystalline silicon with suitable crystallographic and electronic properties that make it a candidate for producing polycrystalline thin films for photovoltaics. AIC can be used to generate crystalline silicon nanowires and other nano-scale structures. Another method of achieving the same result is the use of a laser to heat the silicon locally without heating the underlying substrate beyond some upper temperature limit. An excimer laser or, alternatively, green lasers such as a frequency-doubled Nd:YAG laser is used to heat the amorphous silicon, supplying energy necessary to nucleate grain growth. The laser fluence must be carefully controlled in order to induce crystallization without causing widespread melting. Crystallization of the film occurs as a very small portion of the silicon film is melted and allowed to cool. Ideally, the laser should melt the silicon film through its entire thickness, but not damage the substrate. Toward this end, a layer of silicon dioxide is sometimes added to act as a thermal barrier. This allows the use of substrates that cannot be exposed to the high temperatures of standard annealing, polymers for instance. Polymer-backed solar cells are of interest for seamlessly integrated power production schemes that involve placing photovoltaics on everyday surfaces. A third method for crystallizing amorphous silicon is the use of thermal plasma jet. This strategy is an attempt to alleviate some of the problems associated with laser processing – namely the small region of crystallization and the high cost of the process on a production scale. The plasma torch is a simple piece of equipment that is used to thermally anneal the amorphous silicon. Compared to the laser method, this technique is simpler and more cost effective.. Plasma torch annealing is attractive because the process parameters and equipment dimension can be changed easily to yield varying levels of performance. A high level of crystallization (~90%) can be obtained with this method. Disadvantages include difficulty achieving uniformity in the crystallization of the film. While this method is applied frequently to silicon on a glass substrate, processing temperatures may be too high for polymers. Plasma torch annealing is attractive because the process parameters and equipment dimension can be changed easily to yield varying levels of performance. A high level of crystallization (about 90%) can be obtained with this method. Disadvantages include difficulty achieving uniformity in the crystallization of the film. While this method is applied frequently to silicon on a glass substrate, processing temperatures may be too high for polymers.See also
*List of types of solar cells ''For description and history, see Solar cell''
A solar cell (also called photovoltaic cell or photoelectric cell) is a solid state electrical device that converts the energy of light directly into electricity by the photovoltaic effect, which is ...
References
{{Photovoltaics Silicon, crystalline Silicon solar cells Silicon forms