Furanose on:
[Wikipedia]
[Google]
[Amazon]
 A furanose is a collective term for
A furanose is a collective term for
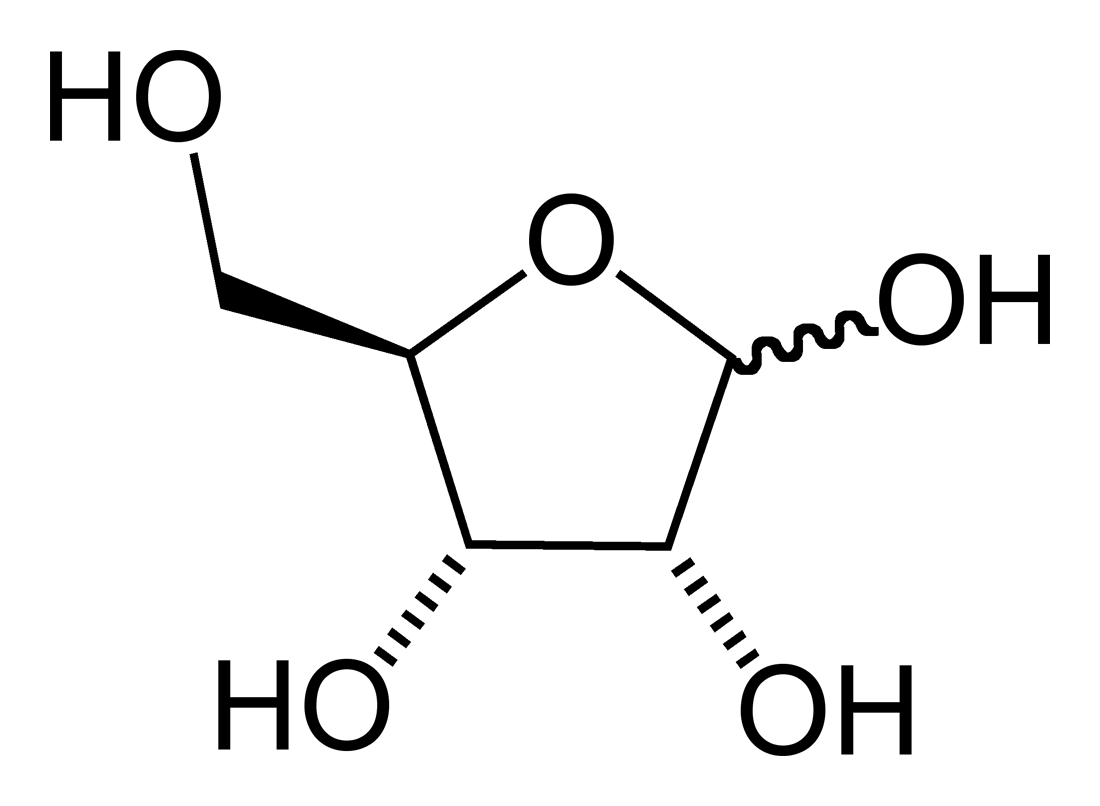 The furanose ring is a cyclic hemiacetal of an aldopentose or a cyclic hemiketal of a ketohexose.
A furanose ring structure consists of four
The furanose ring is a cyclic hemiacetal of an aldopentose or a cyclic hemiketal of a ketohexose.
A furanose ring structure consists of four
carbohydrates
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ma ...
that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle furan, but the furanose ring does not have double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
s.
Structural properties
 The furanose ring is a cyclic hemiacetal of an aldopentose or a cyclic hemiketal of a ketohexose.
A furanose ring structure consists of four
The furanose ring is a cyclic hemiacetal of an aldopentose or a cyclic hemiketal of a ketohexose.
A furanose ring structure consists of four carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
and one oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
atom with the anomeric carbon to the right of the oxygen. The highest numbered chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
carbon (typically to the left of the oxygen in a Haworth projection) determines whether or not the structure has a -configuration or L-configuration. In an -configuration furanose, the substituent on the highest numbered chiral carbon is pointed downwards out of the plane, and in a D-configuration furanose, the highest numbered chiral carbon is facing upwards.
The furanose ring will have either alpha or beta configuration, depending on which direction the anomeric hydroxy group is pointing. In a -configuration furanose, alpha configuration has the hydroxy pointing down, and beta has the hydroxy pointing up. It is the opposite in an -configuration furanose. Typically, the anomeric carbon undergoes mutarotation in solution, and the result is an equilibrium mixture of α and β configurations.
See also
*Pyranose
In organic chemistry, pyranose is a collective term for saccharides that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom (a heterocycle). There may be other carbons external to the ...
References
{{Authority control Carbohydrate chemistry