Fermium on:
[Wikipedia]
[Google]
[Amazon]
Fermium is a synthetic chemical element; it has


 Fermium was first discovered in the fallout from the '
Fermium was first discovered in the fallout from the '
. Retrieved 2 December 2007 Initial examination of the debris from the explosion had shown the production of a new isotope of
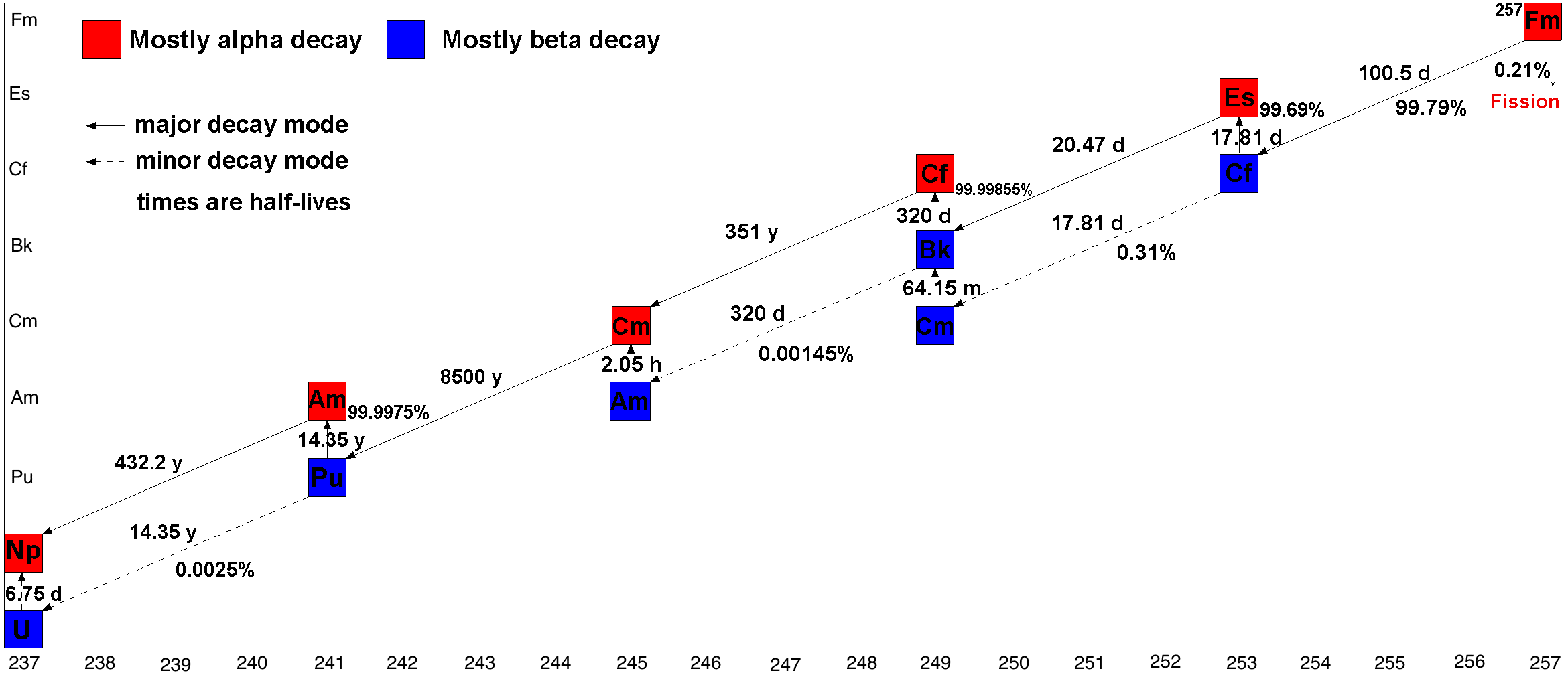 There are 20 isotopes of fermium listed in NUBASE 2016, with atomic weights of 241 to 260, of which Fm is the longest-lived with a
There are 20 isotopes of fermium listed in NUBASE 2016, with atomic weights of 241 to 260, of which Fm is the longest-lived with a
 Fermium is produced by the bombardment of lighter
Fermium is produced by the bombardment of lighter
 The atmospheric results were supplemented by the underground test data accumulated in the 1960s at the Nevada Test Site, as it was hoped that powerful explosions conducted in confined space might result in improved yields and heavier isotopes. Apart from traditional uranium charges, combinations of uranium with americium and thorium have been tried, as well as a mixed plutonium-neptunium charge. They were less successful in terms of yield, which was attributed to stronger losses of heavy isotopes due to enhanced fission rates in heavy-element charges. Isolation of the products was found to be rather problematic, as the explosions were spreading debris through melting and vaporizing rocks under the great depth of 300–600 meters, and drilling to such depth in order to extract the products was both slow and inefficient in terms of collected volumes.Seaborg, p. 40
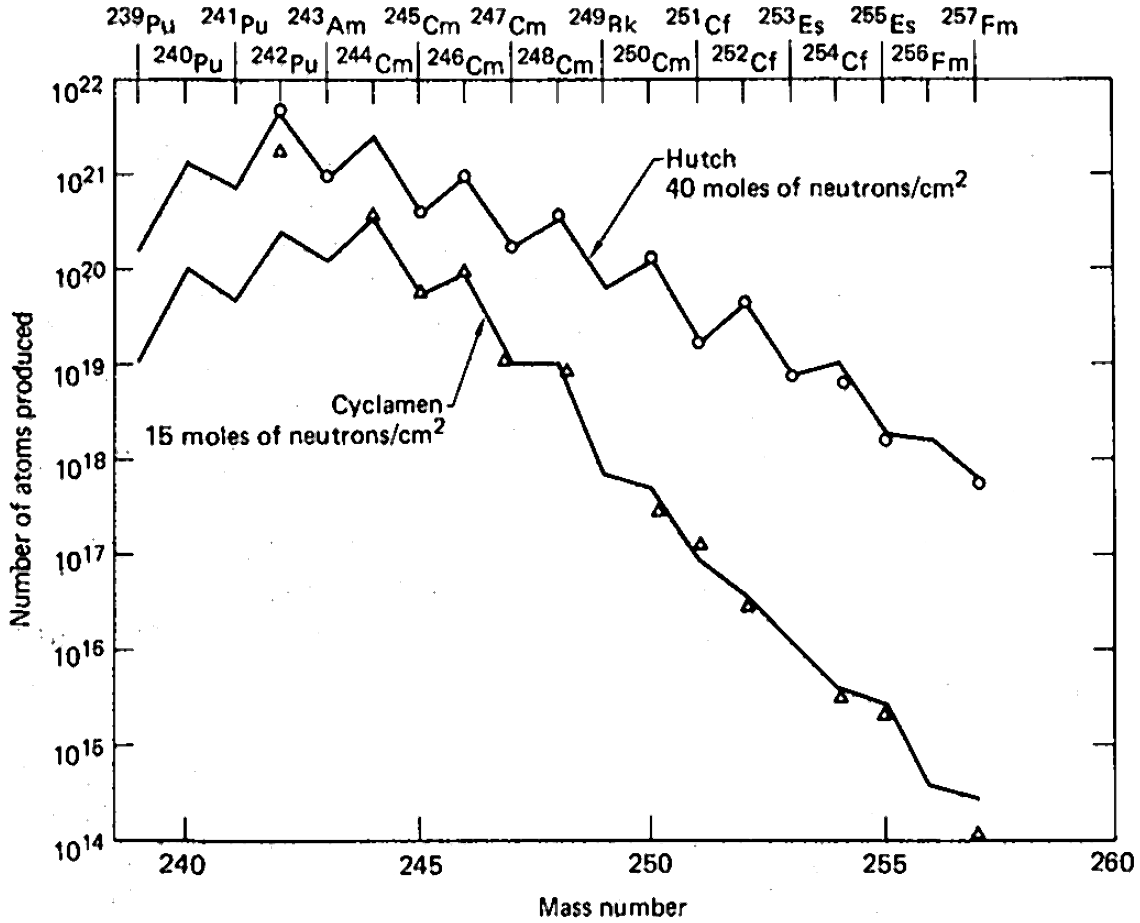
Among the nine underground tests, which were carried between 1962 and 1969 and codenamed Anacostia (5.2 kilotons, 1962), Kennebec (<5 kilotons, 1963), Par (38 kilotons, 1964), Barbel (<20 kilotons, 1964), Tweed (<20 kilotons, 1965), Cyclamen (13 kilotons, 1966), Kankakee (20-200 kilotons, 1966), Vulcan (25 kilotons, 1966) and Hutch (20-200 kilotons, 1969), the last one was most powerful and had the highest yield of transuranium elements. In the dependence on the atomic mass number, the yield showed a saw-tooth behavior with the lower values for odd isotopes, due to their higher fission rates. The major practical problem of the entire proposal, however, was collecting the radioactive debris dispersed by the powerful blast. Aircraft filters adsorbed only about 4 of the total amount and collection of tons of corals at Enewetak Atoll increased this fraction by only two orders of magnitude. Extraction of about 500 kilograms of underground rocks 60 days after the Hutch explosion recovered only about 10 of the total charge. The amount of transuranium elements in this 500-kg batch was only 30 times higher than in a 0.4 kg rock picked up 7 days after the test. This observation demonstrated the highly nonlinear dependence of the transuranium elements yield on the amount of retrieved radioactive rock.Seaborg, p. 43 In order to accelerate sample collection after the explosion, shafts were drilled at the site not after but before the test, so that the explosion would expel radioactive material from the epicenter, through the shafts, to collecting volumes near the surface. This method was tried in the Anacostia and Kennebec tests and instantly provided hundreds of kilograms of material, but with actinide concentrations 3 times lower than in samples obtained after drilling; whereas such a method could have been efficient in scientific studies of short-lived isotopes, it could not improve the overall collection efficiency of the produced actinides.Seaborg, p. 44
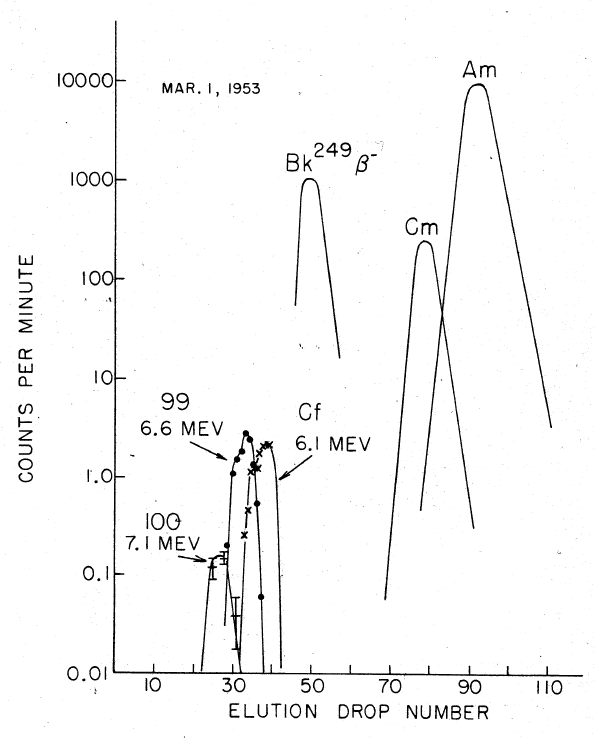
Though no new elements (apart from einsteinium and fermium) could be detected in the nuclear test debris, and the total yields of transuranium elements were disappointingly low, these tests did provide significantly higher amounts of rare heavy isotopes than previously available in laboratories. For example, 6 atoms of Fm could be recovered after the Hutch detonation. They were then used in the studies of thermal-neutron induced fission of Fm and in discovery of a new fermium isotope Fm. Also, the rare isotope Cm was synthesized in large quantities, which is very difficult to produce in nuclear reactors from its progenitor Cm; the half-life of Cm (64 minutes) is much too short for months-long reactor irradiations, but is very "long" on the explosion timescale.Seaborg, p. 47
The atmospheric results were supplemented by the underground test data accumulated in the 1960s at the Nevada Test Site, as it was hoped that powerful explosions conducted in confined space might result in improved yields and heavier isotopes. Apart from traditional uranium charges, combinations of uranium with americium and thorium have been tried, as well as a mixed plutonium-neptunium charge. They were less successful in terms of yield, which was attributed to stronger losses of heavy isotopes due to enhanced fission rates in heavy-element charges. Isolation of the products was found to be rather problematic, as the explosions were spreading debris through melting and vaporizing rocks under the great depth of 300–600 meters, and drilling to such depth in order to extract the products was both slow and inefficient in terms of collected volumes.Seaborg, p. 40
Among the nine underground tests, which were carried between 1962 and 1969 and codenamed Anacostia (5.2 kilotons, 1962), Kennebec (<5 kilotons, 1963), Par (38 kilotons, 1964), Barbel (<20 kilotons, 1964), Tweed (<20 kilotons, 1965), Cyclamen (13 kilotons, 1966), Kankakee (20-200 kilotons, 1966), Vulcan (25 kilotons, 1966) and Hutch (20-200 kilotons, 1969), the last one was most powerful and had the highest yield of transuranium elements. In the dependence on the atomic mass number, the yield showed a saw-tooth behavior with the lower values for odd isotopes, due to their higher fission rates. The major practical problem of the entire proposal, however, was collecting the radioactive debris dispersed by the powerful blast. Aircraft filters adsorbed only about 4 of the total amount and collection of tons of corals at Enewetak Atoll increased this fraction by only two orders of magnitude. Extraction of about 500 kilograms of underground rocks 60 days after the Hutch explosion recovered only about 10 of the total charge. The amount of transuranium elements in this 500-kg batch was only 30 times higher than in a 0.4 kg rock picked up 7 days after the test. This observation demonstrated the highly nonlinear dependence of the transuranium elements yield on the amount of retrieved radioactive rock.Seaborg, p. 43 In order to accelerate sample collection after the explosion, shafts were drilled at the site not after but before the test, so that the explosion would expel radioactive material from the epicenter, through the shafts, to collecting volumes near the surface. This method was tried in the Anacostia and Kennebec tests and instantly provided hundreds of kilograms of material, but with actinide concentrations 3 times lower than in samples obtained after drilling; whereas such a method could have been efficient in scientific studies of short-lived isotopes, it could not improve the overall collection efficiency of the produced actinides.Seaborg, p. 44
Though no new elements (apart from einsteinium and fermium) could be detected in the nuclear test debris, and the total yields of transuranium elements were disappointingly low, these tests did provide significantly higher amounts of rare heavy isotopes than previously available in laboratories. For example, 6 atoms of Fm could be recovered after the Hutch detonation. They were then used in the studies of thermal-neutron induced fission of Fm and in discovery of a new fermium isotope Fm. Also, the rare isotope Cm was synthesized in large quantities, which is very difficult to produce in nuclear reactors from its progenitor Cm; the half-life of Cm (64 minutes) is much too short for months-long reactor irradiations, but is very "long" on the explosion timescale.Seaborg, p. 47
 The chemistry of fermium has only been studied in solution using tracer techniques, and no solid compounds have been prepared. Under normal conditions, fermium exists in solution as the Fm3+ ion, which has a hydration number of 16.9 and an
The chemistry of fermium has only been studied in solution using tracer techniques, and no solid compounds have been prepared. Under normal conditions, fermium exists in solution as the Fm3+ ion, which has a hydration number of 16.9 and an
Fermium, Mendelevium, Nobelium, and Lawrencium
in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Hrsg.): ''The Chemistry of the Actinide and Transactinide Elements'', Springer, Dordrecht 2006; , p. 1621–1651; . * Seaborg, Glenn T. (ed.) (1978)
Proceedings of the Symposium Commemorating the 25th Anniversary of Elements 99 and 100
', 23 January 1978, Report LBL-7701 * '' Gmelins Handbuch der anorganischen Chemie'', System Nr. 71, Transurane: Teil A 1 II, p. 19–20; Teil A 2, p. 47; Teil B 1, p. 84.
Fermium
at ''
symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
Fm and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
100. It is an actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
and the heaviest element that can be formed by neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
bombardment of lighter elements, and hence the last element that can be prepared in macroscopic quantities, although pure fermium metal has not been prepared yet. A total of 20 isotopes are known, with 257Fm being the longest-lived with a half-life of 100.5 days.
Fermium was discovered in the debris of the first hydrogen bomb
A thermonuclear weapon, fusion weapon or hydrogen bomb (H-bomb) is a second-generation nuclear weapon design. Its greater sophistication affords it vastly greater destructive power than first-generation nuclear bombs, a more compact size, a lo ...
explosion in 1952, and named after Enrico Fermi, one of the pioneers of nuclear physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies th ...
. Its chemistry is typical for the late actinides, with a preponderance of the +3 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
but also an accessible +2 oxidation state. Owing to the small amounts of produced fermium and all of its isotopes having relatively short half-lives, there are currently no uses for it outside basic scientific research.
Discovery


 Fermium was first discovered in the fallout from the '
Fermium was first discovered in the fallout from the 'Ivy Mike
Ivy Mike was the code name, codename given to the first full-scale test of a Thermonuclear weapon, thermonuclear device, in which a significant fraction of the explosive nuclear weapon yield, yield comes from nuclear fusion.
Ivy Mike was detona ...
' nuclear test (1 November 1952), the first successful test of a hydrogen bomb.Fermium – National Research Council Canada. Retrieved 2 December 2007 Initial examination of the debris from the explosion had shown the production of a new isotope of
plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
, : this could only have formed by the absorption of six neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s by a uranium-238 nucleus followed by two β− decays. At the time, the absorption of neutrons by a heavy nucleus was thought to be a rare process, but the identification of raised the possibility that still more neutrons could have been absorbed by the uranium nuclei, leading to new elements.
Element 99 ( einsteinium) was quickly discovered on filter papers which had been flown through clouds from the explosion (the same sampling technique that had been used to discover ). It was then identified in December 1952 by Albert Ghiorso
Albert Ghiorso (July 15, 1915 – December 26, 2010) was an American nuclear scientist and co-discoverer of a record 12 chemical elements on the periodic table. His research career spanned six decades, from the early 1940s to the late 1990s.
Biog ...
and co-workers at the University of California at Berkeley. They discovered the isotope 253Es (half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
) that was made by the capture of 15 neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s by uranium-238 nuclei – which then underwent seven successive beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
s:
Some 238U atoms, however, could capture another amount of neutrons (most likely, 16 or 17). The discovery of fermium () required more material, as the yield was expected to be at least an order of magnitude lower than that of element 99, and so contaminated coral from the Enewetak atoll (where the test had taken place) was shipped to the University of California Radiation Laboratory in
Berkeley, California
Berkeley ( ) is a city on the eastern shore of San Francisco Bay in northern Alameda County, California, United States. It is named after the 18th-century Anglo-Irish bishop and philosopher George Berkeley. It borders the cities of Oakland, Cali ...
, for processing and analysis. About two months after the test, a new component was isolated emitting high-energy α-particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produc ...
s () with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of about a day. With such a short half-life, it could only arise from the β− decay of an isotope of einsteinium, and so had to be an isotope of the new element 100: it was quickly identified as 255Fm ().
The discovery of the new elements, and the new data on neutron capture, was initially kept secret on the orders of the U.S. military until 1955 due to Cold War
The Cold War was a period of global Geopolitics, geopolitical rivalry between the United States (US) and the Soviet Union (USSR) and their respective allies, the capitalist Western Bloc and communist Eastern Bloc, which lasted from 1947 unt ...
tensions. Nevertheless, the Berkeley team was able to prepare elements 99 and 100 by civilian means, through the neutron bombardment of plutonium-239, and published this work in 1954 with the disclaimer that it was not the first studies that had been carried out on the elements. The "Ivy Mike" studies were declassified and published in 1955.
The Berkeley team had been worried that another group might discover lighter isotopes of element 100 through ion-bombardment techniques before they could publish their classified research, and this proved to be the case. A group at the Nobel Institute for Physics in Stockholm independently discovered the element, producing an isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
later confirmed to be 250Fm () by bombarding a target with oxygen-16 ions, and published their work in May 1954. Nevertheless, the priority of the Berkeley team was generally recognized, and with it the prerogative to name the new element in honour of Enrico Fermi, the developer of the first artificial self-sustained nuclear reactor. Fermi was still alive when the name was proposed, but had died by the time it became official.
Isotopes
half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 100.5 days. Fm has a half-life of 3 days, while Fm of 5.3 h, Fm of 25.4 h, Fm of 3.2 h, Fm of 20.1 h, and Fm of 2.6 hours. All the remaining ones have half-lives ranging from 30 minutes to less than a millisecond.
The neutron capture product of fermium-257, Fm, undergoes spontaneous fission with a half-life of just 370(14) microseconds; Fm and Fm also undergo spontaneous fission (''t''1/2 = 1.5(3) s and 4 ms respectively). This means that neutron capture cannot be used to create nuclide
Nuclides (or nucleides, from nucleus, also known as nuclear species) are a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by the A ...
s with a mass number
The mass number (symbol ''A'', from the German word: ''Atomgewicht'', "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is appro ...
greater than 257, unless carried out in a nuclear explosion. As Fm alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
s to Cf, and no known fermium isotopes undergo beta minus decay to the next element, mendelevium
Mendelevium is a synthetic chemical element; it has symbol Md ( formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced ...
, fermium is also the last element that can be synthesized by neutron-capture. Because of this impediment in forming heavier isotopes, these short-lived isotopes Fm constitute the "fermium gap."
Occurrence
Production
 Fermium is produced by the bombardment of lighter
Fermium is produced by the bombardment of lighter actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
s with neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s in a nuclear reactor. Fermium-257 is the heaviest isotope that is obtained via neutron capture, and can only be produced in picogram quantities. The major source is the 85 MW High Flux Isotope Reactor (HFIR) at the Oak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a federally funded research and development centers, federally funded research and development center in Oak Ridge, Tennessee, United States. Founded in 1943, the laboratory is sponsored by the United Sta ...
in Tennessee
Tennessee (, ), officially the State of Tennessee, is a landlocked U.S. state, state in the Southeastern United States, Southeastern region of the United States. It borders Kentucky to the north, Virginia to the northeast, North Carolina t ...
, USA, which is dedicated to the production of transcurium (''Z'' > 96) elements. Lower mass fermium isotopes are available in greater quantities, though these isotopes (254Fm and 255Fm) are comparatively short-lived. In a "typical processing campaign" at Oak Ridge, tens of grams of curium are irradiated to produce decigram quantities of californium
Californium is a synthetic chemical element; it has symbol Cf and atomic number 98. It was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) by bombarding curium with al ...
, milligram quantities of berkelium and einsteinium, and picogram quantities of fermium. However, nanogram quantities of fermium can be prepared for specific experiments. The quantities of fermium produced in 20–200 kiloton thermonuclear explosions is believed to be of the order of milligrams, although it is mixed in with a huge quantity of debris; 4.0 picograms of 257Fm was recovered from 10 kilograms of debris from the " Hutch" test (16 July 1969). The Hutch experiment produced an estimated total of 250 micrograms of 257Fm.
After production, the fermium must be separated from other actinides and from lanthanide fission products. This is usually achieved by ion-exchange chromatography, with the standard process using a cation exchanger such as Dowex 50 or TEVA eluted with a solution of ammonium α-hydroxyisobutyrate. Smaller cations form more stable complexes with the α-hydroxyisobutyrate anion, and so are preferentially eluted from the column. A rapid fractional crystallization method has also been described.
Although the most stable isotope of fermium is 257Fm, with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 100.5 days, most studies are conducted on 255Fm (''t''1/2 = 20.07(7) hours), since this isotope can be easily isolated as required as the decay product of 255Es (''t''1/2 = 39.8(12) days).
Synthesis in nuclear explosions
The analysis of the debris at the 10- megaton ''Ivy Mike'' nuclear test was a part of long-term project, one of the goals of which was studying the efficiency of production of transuranium elements in high-power nuclear explosions. The motivation for these experiments was as follows: synthesis of such elements from uranium requires multiple neutron capture. The probability of such events increases with the neutron flux, and nuclear explosions are the most powerful neutron sources, providing densities on the order 10 neutrons/cm within a microsecond, i.e. about 10 neutrons/(cm·s). For comparison, the flux of the HFIR reactor is 5 neutrons/(cm·s). A dedicated laboratory was set up right at Enewetak Atoll for preliminary analysis of debris, as some isotopes could have decayed by the time the debris samples reached the U.S. The laboratory was receiving samples for analysis, as soon as possible, from airplanes equipped with paper filters which flew over the atoll after the tests. Whereas it was hoped to discover new chemical elements heavier than fermium, those were not found after a series of megaton explosions conducted between 1954 and 1956 at the atoll.Seaborg, p. 39 The atmospheric results were supplemented by the underground test data accumulated in the 1960s at the Nevada Test Site, as it was hoped that powerful explosions conducted in confined space might result in improved yields and heavier isotopes. Apart from traditional uranium charges, combinations of uranium with americium and thorium have been tried, as well as a mixed plutonium-neptunium charge. They were less successful in terms of yield, which was attributed to stronger losses of heavy isotopes due to enhanced fission rates in heavy-element charges. Isolation of the products was found to be rather problematic, as the explosions were spreading debris through melting and vaporizing rocks under the great depth of 300–600 meters, and drilling to such depth in order to extract the products was both slow and inefficient in terms of collected volumes.Seaborg, p. 40
Among the nine underground tests, which were carried between 1962 and 1969 and codenamed Anacostia (5.2 kilotons, 1962), Kennebec (<5 kilotons, 1963), Par (38 kilotons, 1964), Barbel (<20 kilotons, 1964), Tweed (<20 kilotons, 1965), Cyclamen (13 kilotons, 1966), Kankakee (20-200 kilotons, 1966), Vulcan (25 kilotons, 1966) and Hutch (20-200 kilotons, 1969), the last one was most powerful and had the highest yield of transuranium elements. In the dependence on the atomic mass number, the yield showed a saw-tooth behavior with the lower values for odd isotopes, due to their higher fission rates. The major practical problem of the entire proposal, however, was collecting the radioactive debris dispersed by the powerful blast. Aircraft filters adsorbed only about 4 of the total amount and collection of tons of corals at Enewetak Atoll increased this fraction by only two orders of magnitude. Extraction of about 500 kilograms of underground rocks 60 days after the Hutch explosion recovered only about 10 of the total charge. The amount of transuranium elements in this 500-kg batch was only 30 times higher than in a 0.4 kg rock picked up 7 days after the test. This observation demonstrated the highly nonlinear dependence of the transuranium elements yield on the amount of retrieved radioactive rock.Seaborg, p. 43 In order to accelerate sample collection after the explosion, shafts were drilled at the site not after but before the test, so that the explosion would expel radioactive material from the epicenter, through the shafts, to collecting volumes near the surface. This method was tried in the Anacostia and Kennebec tests and instantly provided hundreds of kilograms of material, but with actinide concentrations 3 times lower than in samples obtained after drilling; whereas such a method could have been efficient in scientific studies of short-lived isotopes, it could not improve the overall collection efficiency of the produced actinides.Seaborg, p. 44
Though no new elements (apart from einsteinium and fermium) could be detected in the nuclear test debris, and the total yields of transuranium elements were disappointingly low, these tests did provide significantly higher amounts of rare heavy isotopes than previously available in laboratories. For example, 6 atoms of Fm could be recovered after the Hutch detonation. They were then used in the studies of thermal-neutron induced fission of Fm and in discovery of a new fermium isotope Fm. Also, the rare isotope Cm was synthesized in large quantities, which is very difficult to produce in nuclear reactors from its progenitor Cm; the half-life of Cm (64 minutes) is much too short for months-long reactor irradiations, but is very "long" on the explosion timescale.Seaborg, p. 47
The atmospheric results were supplemented by the underground test data accumulated in the 1960s at the Nevada Test Site, as it was hoped that powerful explosions conducted in confined space might result in improved yields and heavier isotopes. Apart from traditional uranium charges, combinations of uranium with americium and thorium have been tried, as well as a mixed plutonium-neptunium charge. They were less successful in terms of yield, which was attributed to stronger losses of heavy isotopes due to enhanced fission rates in heavy-element charges. Isolation of the products was found to be rather problematic, as the explosions were spreading debris through melting and vaporizing rocks under the great depth of 300–600 meters, and drilling to such depth in order to extract the products was both slow and inefficient in terms of collected volumes.Seaborg, p. 40
Among the nine underground tests, which were carried between 1962 and 1969 and codenamed Anacostia (5.2 kilotons, 1962), Kennebec (<5 kilotons, 1963), Par (38 kilotons, 1964), Barbel (<20 kilotons, 1964), Tweed (<20 kilotons, 1965), Cyclamen (13 kilotons, 1966), Kankakee (20-200 kilotons, 1966), Vulcan (25 kilotons, 1966) and Hutch (20-200 kilotons, 1969), the last one was most powerful and had the highest yield of transuranium elements. In the dependence on the atomic mass number, the yield showed a saw-tooth behavior with the lower values for odd isotopes, due to their higher fission rates. The major practical problem of the entire proposal, however, was collecting the radioactive debris dispersed by the powerful blast. Aircraft filters adsorbed only about 4 of the total amount and collection of tons of corals at Enewetak Atoll increased this fraction by only two orders of magnitude. Extraction of about 500 kilograms of underground rocks 60 days after the Hutch explosion recovered only about 10 of the total charge. The amount of transuranium elements in this 500-kg batch was only 30 times higher than in a 0.4 kg rock picked up 7 days after the test. This observation demonstrated the highly nonlinear dependence of the transuranium elements yield on the amount of retrieved radioactive rock.Seaborg, p. 43 In order to accelerate sample collection after the explosion, shafts were drilled at the site not after but before the test, so that the explosion would expel radioactive material from the epicenter, through the shafts, to collecting volumes near the surface. This method was tried in the Anacostia and Kennebec tests and instantly provided hundreds of kilograms of material, but with actinide concentrations 3 times lower than in samples obtained after drilling; whereas such a method could have been efficient in scientific studies of short-lived isotopes, it could not improve the overall collection efficiency of the produced actinides.Seaborg, p. 44
Though no new elements (apart from einsteinium and fermium) could be detected in the nuclear test debris, and the total yields of transuranium elements were disappointingly low, these tests did provide significantly higher amounts of rare heavy isotopes than previously available in laboratories. For example, 6 atoms of Fm could be recovered after the Hutch detonation. They were then used in the studies of thermal-neutron induced fission of Fm and in discovery of a new fermium isotope Fm. Also, the rare isotope Cm was synthesized in large quantities, which is very difficult to produce in nuclear reactors from its progenitor Cm; the half-life of Cm (64 minutes) is much too short for months-long reactor irradiations, but is very "long" on the explosion timescale.Seaborg, p. 47
Natural occurrence
Because of the short half-life of all known isotopes of fermium, any primordial fermium, that is fermium present on Earth during its formation, has decayed by now. Synthesis of fermium from naturally occurring uranium and thorium in the Earth's crust requires multiple neutron captures, which is extremely unlikely. Therefore, most fermium is produced on Earth in laboratories, high-power nuclear reactors, or in nuclear tests, and is present for only a few months afterward. The transuranic elementsamericium
Americium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element e ...
to fermium did occur naturally in the natural nuclear fission reactor at Oklo, but no longer do so.
Chemistry
 The chemistry of fermium has only been studied in solution using tracer techniques, and no solid compounds have been prepared. Under normal conditions, fermium exists in solution as the Fm3+ ion, which has a hydration number of 16.9 and an
The chemistry of fermium has only been studied in solution using tracer techniques, and no solid compounds have been prepared. Under normal conditions, fermium exists in solution as the Fm3+ ion, which has a hydration number of 16.9 and an acid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative property, quantitative measure of the acid strength, strength of an acid in Solution (chemistry), solution. I ...
of 1.6 (p''K'' = 3.8). Fm forms complexes with a wide variety of organic ligands with hard donor atoms such as oxygen, and these complexes are usually more stable than those of the preceding actinides. It also forms anionic complexes with ligands such as chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
or nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
and, again, these complexes appear to be more stable than those formed by einsteinium or californium
Californium is a synthetic chemical element; it has symbol Cf and atomic number 98. It was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) by bombarding curium with al ...
. It is believed that the bonding in the complexes of the later actinides is mostly ionic in character: the Fm ion is expected to be smaller than the preceding An ions because of the higher effective nuclear charge
In atomic physics, the effective nuclear charge of an electron in a multi-electron atom or ion is the number of elementary charges (e) an electron experiences by the nucleus. It is denoted by ''Z''eff. The term "effective" is used because the shi ...
of fermium, and hence fermium would be expected to form shorter and stronger metal–ligand bonds.
Fermium(III) can be fairly easily reduced to fermium(II), for example with samarium(II) chloride, with which fermium(II) coprecipitates. In the precipitate, the compound fermium(II) chloride (FmCl) was produced, though it was not purified or studied in isolation. The electrode potential
An electrode is an electrical conductor used to make contact with a nonmetallic part of a Electronic circuit, circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can c ...
has been estimated to be similar to that of the ytterbium(III)/(II) couple, or about −1.15 V with respect to the standard hydrogen electrode
In electrochemistry, the standard hydrogen electrode (abbreviated SHE), is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials. Its absolute electrode potential is estimated to be at 25 ° ...
, a value which agrees with theoretical calculations. The Fm/Fm couple has an electrode potential of −2.37(10) V based on polarographic measurements.
Toxicity
Though few people come in contact with fermium, the International Commission on Radiological Protection has set annual exposure limits for the two most stable isotopes. For fermium-253, the ingestion limit was set at 10becquerel
The becquerel (; symbol: Bq) is the unit of radioactivity in the International System of Units (SI). One becquerel is defined as an activity of one per second, on average, for aperiodic activity events referred to a radionuclide. For applicatio ...
s (1 Bq equals one decay per second), and the inhalation limit at 10 Bq; for fermium-257, at 10 Bq and 4,000 Bq respectively.
Notes and references
Notes
References
Further reading
* Robert J. SilvaFermium, Mendelevium, Nobelium, and Lawrencium
in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Hrsg.): ''The Chemistry of the Actinide and Transactinide Elements'', Springer, Dordrecht 2006; , p. 1621–1651; . * Seaborg, Glenn T. (ed.) (1978)
Proceedings of the Symposium Commemorating the 25th Anniversary of Elements 99 and 100
', 23 January 1978, Report LBL-7701 * '' Gmelins Handbuch der anorganischen Chemie'', System Nr. 71, Transurane: Teil A 1 II, p. 19–20; Teil A 2, p. 47; Teil B 1, p. 84.
External links
Fermium
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
{{Authority control
Chemical elements
Chemical elements with face-centered cubic structure
Actinides
Synthetic elements