Experiments and Observations on Different Kinds of Air on:
[Wikipedia]
[Google]
[Amazon]
''Experiments and Observations on Different Kinds of Air'' (1774–86) is a six-volume work published by 18th-century British
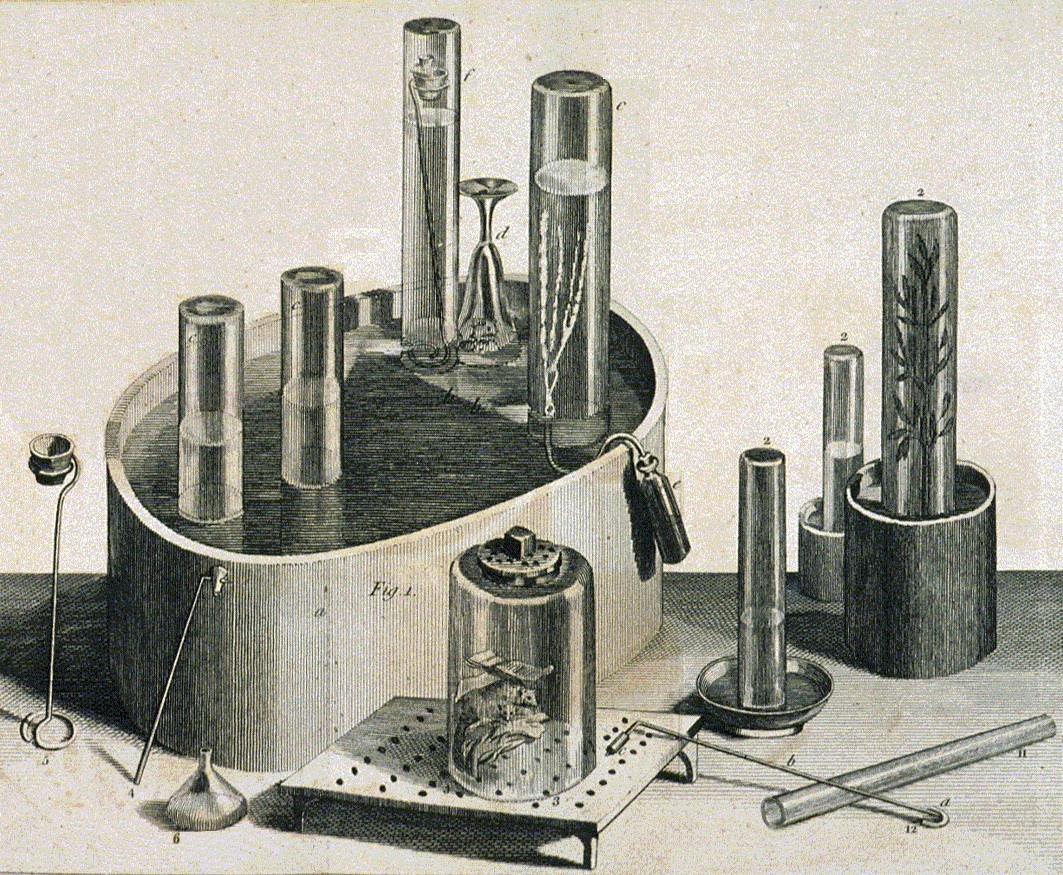 Priestley's first volume of ''Experiments and Observations on Different Kinds of Air'' outlined several important discoveries: experiments that would eventually lead to the discovery of
Priestley's first volume of ''Experiments and Observations on Different Kinds of Air'' outlined several important discoveries: experiments that would eventually lead to the discovery of
polymath
A polymath ( el, πολυμαθής, , "having learned much"; la, homo universalis, "universal human") is an individual whose knowledge spans a substantial number of subjects, known to draw on complex bodies of knowledge to solve specific pro ...
Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted ...
which reports a series of his experiments on "airs" or gases, most notably his discovery of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
gas (which he called "dephlogisticated air").
Airs
While working as a companion forLord Shelburne
William Petty Fitzmaurice, 1st Marquess of Lansdowne, (2 May 17377 May 1805; known as the Earl of Shelburne between 1761 and 1784, by which title he is generally known to history), was an Irish-born British Whig statesman who was the first ...
, Priestley had a great deal of free time to engage in scientific investigations. The Earl even set up a laboratory for him. Priestley's experiments during his years in Calne
Calne () is a town and civil parish in Wiltshire, southwestern England,OS Explorer Map 156, Chippenham and Bradford-on-Avon Scale: 1:25 000.Publisher: Ordnance Survey A2 edition (2007). at the northwestern extremity of the North Wessex Downs ...
were almost entirely confined to "airs" and from this work emerged his most important scientific texts: the six volumes of ''Experiments and Observations on Different Kinds of Air''. These experiments helped repudiate the last vestiges of the theory of four elements; as one early biographer writes: "taken collectively, riestleydid more than those of any one of his contemporaries to uproot and destroy the only generalisation by which his immediate predecessors had sought to group and connect the phenomena of chemistry", however "he was wholly unable to perceive this fact." Priestley's work on "airs" is not easily classified. As historian of science Simon Schaffer points out, it "has been seen as a branch of physics, or chemistry, or natural philosophy, or some highly idiosyncratic version of Priestley's own invention." Also, the volumes were both a scientific and a political enterprise for Priestley; he argued in them that science could destroy "undue and usurped authority," writing that the government has "reason to tremble even at an air pump or an electrical machine."
 Priestley's first volume of ''Experiments and Observations on Different Kinds of Air'' outlined several important discoveries: experiments that would eventually lead to the discovery of
Priestley's first volume of ''Experiments and Observations on Different Kinds of Air'' outlined several important discoveries: experiments that would eventually lead to the discovery of photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in ...
and the discovery of several airs: "nitrous air" (nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its ...
, NO), "vapor of spirit of salt" (later called "acid air" or "marine acid air"; anhydrous hydrochloric acid, HCl), "alkaline air" (ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
, NH3), "diminished" or "dephlogisticated nitrous air" (nitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or nos, is a chemical compound, an oxide of nitrogen with the formula . At room temperature, it is a colourless non-flammable gas, and ha ...
, N2O), and "dephlogisticated air" (oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
, O2). Priestley also developed the "nitrous air test", which tested for the "goodness of air": using a "pneumatic trough", he would mix nitrous air with a test sample, over water or mercury, and measure the decrease in volume—the principle of eudiometry.Fruton, 20; 29 After a small history of the study of airs, he explained his own experiments in an open and sincere style: "whatever he knows or thinks he tells: doubts, perplexities, blunders are set down with the most refreshing candour." He also invented and described cheap and easy-to-assemble experimental apparatus. His colleagues therefore believed that they could easily reproduce Priestley's experiments to verify them or to answer the questions that had puzzled him.
Although many of his results puzzled him, Priestley used phlogiston theory
The phlogiston theory is a superseded scientific theory that postulated the existence of a fire-like element called phlogiston () contained within combustible bodies and released during combustion. The name comes from the Ancient Greek (''bur ...
to resolve the difficulties. This theory, however, led him to conclude that there were only three types of "air": "fixed", "alkaline", and "acid". Priestley ignored the burgeoning chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, proper ...
of his day, indeed dismissing it in these volumes. Instead, he focused on gases and the "changes in their sensible properties", as had natural philosophers before him. He isolated carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
(CO) but seems not to have realised that it was a separate "air" from the others that he had discovered.
Discovery of oxygen
After the publication of the first volume of ''Experiments and Observations'', Priestley undertook another set of experiments. In August 1774 he isolated an "air" that appeared to be completely new, but he did not have an opportunity to pursue the matter because he was about to tour Europe with Shelburne. While in Paris, however, Priestley managed to replicate the experiment for others, includingAntoine Lavoisier
Antoine-Laurent de Lavoisier ( , ; ; 26 August 17438 May 1794),
CNRS (
CNRS (
sulphur dioxide, SO2). In March he wrote to several people regarding the new "air" that he had discovered several months earlier. One of these letters was read aloud to the
File:Priestly-1.jpg, Volumes I-III of ''Experiments and Observations on Different Kinds of Air'' (1774, 1775, 1779)
File:Priestly-5.jpg, Preface to volume I of ''Experiments and Observations on Different Kinds of Air'' (1774)
File:Priestly-6.jpg, Introduction to volume I of ''Experiments and Observations on Different Kinds of Air'' (1774)
File:Priestly-7.jpg, First page of volume I of ''Experiments and Observations on Different Kinds of Air'' (1774)
''Directions for Impregnating Water with Fixed Air''
London, 1772.
''Experiments and Observations on Different Kinds of Air, Vol.1''
London, 1774.
''Experiments and Observations on Different Kinds of Air, Vol.2''
London, 1775. * ''Experiments and Observations on Different Kinds of Air, Vol.3''. London, 1777. * ''Experiments and Observations relating to various Branches of Natural Philosophy, Vol.1''. 'Experiments and Observations on Different Kinds of Air, Vol.4'' London, 1779.
''Experiments and Observations relating to various Branches of Natural Philosophy, Vol.2''
'Experiments and Observations on Different Kinds of Air, Vol.5'' Birmingham, 1781. * ''Experiments Relating to Phlogiston''. London, 1784. * ''Experiments and Observations relating to various Branches of Natural Philosophy, Vol.3''. 'Experiments and Observations on Different Kinds of Air, Vol.6'' Birmingham, 1786.
''Experiments and Observations on Different Kinds of Air, Vol.1–6''
''In 3 volumes, being the former 6 abridged and methodised, with many additions''. Birmingham, 1790. Books from 1791–1803: * ''Experiments on the Generation of Air from Water; to which are prefixed, Experiments relating to the Decomposition of Dephlogisticated and Inflammable Air''. London, 1793.
''Heads of Lectures on a Course of Experimental Philosophy; delivered at the New College in Hackney''
irst 10 of 36 lectures are about Airs London, 1794. * ''Considerations on the Doctrine of Phlogiston and the Decomposition of Water''. Philadelphia, 1796. * ''Experiments and Observations relating to the Analysis of Atmospherical Air; also farther Experiments relating to the Generation of Air from Water.'' [Red before the American Philosophical Society, Feb.5th and 19th in 1796, and printed in their Transactions. To which are added, ''Considerations on the Doctrine of Phlogiston, and the Decomposition of Water'', addressed to Messrs. Berthollet &c]. London, 1796. * ''Considerations on the Doctrine of Phlogiston and the Decomposition of Water, Part II''. Philadelphia, 1797.Schofield, Robert E. ''The Enlightened Joseph Priestley: A Study of His Life and Work from 1773 to 1804''. University Park: Pennsylvania State University Press (2004). . * ''Doctrine of Phlogiston established and that of the Composition of Water refuted''. Northumberland, 1800. * ''Doctrine of Phlogiston established, with Observations on the Conversion of Iron into Steel, in a Letter to Mr. Nicholson''. Printed in 1803.
full text
(1772) *
full text
(2nd edition, 1774) {{DEFAULTSORT:Experiments And Observations On Different Kinds Of Air 1774 books Science books Books by Joseph Priestley 1774 in science Oxygen
Royal Society
The Royal Society, formally The Royal Society of London for Improving Natural Knowledge, is a learned society and the United Kingdom's national academy of sciences. The society fulfils a number of roles: promoting science and its benefits, re ...
, and he published a paper in ''Philosophical Transactions
''Philosophical Transactions of the Royal Society'' is a scientific journal published by the Royal Society. In its earliest days, it was a private venture of the Royal Society's secretary. It was established in 1665, making it the first journa ...
'' titled "An Account of further Discoveries in Air." Priestley called the new substance "dephlogisticated air" and described it as "five or six times better than common air for the purpose of respiration, inflammation, and, I believe, every other use of common atmospherical air." He had discovered oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
gas (O2). As revised for ''Experiments and Observations'', his paper begins:
The contents of this section will furnish a very striking illustration of the truth of a remark which I have more than once made in my aturalphilosophical writings … that more is owing to what we call ''chance''—that is, philosophically speaking, to the observations of ''events rising from unknown causes'' than to any proper ''design'' or preconceived ''theory'' in this business. … For my own part, I will frankly acknowledge that at the commencement of my experiments recited in this section I was so far from having formed any hypothesis that led to the discoveries I made in pursuing them that they would have appeared very improbable to me had I been told of them; and when the decisive facts did at length obtrude themselves upon my notice it was very slowly, and with great hesitation, that I yielded to the evidence of my senses. mphasis Priestley'sPriestley assembled his oxygen paper and several others into a second volume of ''Experiments and Observations on Air'' and published it in 1776. He does not emphasise his discovery of "dephlogisticated air" (leaving it to Part III of the volume) but instead argues in the preface how important such discoveries are to rational religion. His paper narrates the discovery chronologically, relating the long delays between experiments and his initial puzzlements. Thus, it is difficult to determine when exactly Priestley "discovered" oxygen. Such dating is significant as Lavoisier and Swedish pharmacist
Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydr ...
both have strong claims to the discovery of oxygen as well, Scheele having been first to isolate the gas (although he published after Priestley) and Lavoisier having been first to describe it as purified "air itself entire without alteration" (not "dephlogisticated air").Kuhn, 53–55.Scientific work on Airs
In this section, a list of all Priestley's scientific books on Airs has been compiled. The list doesn't include any of the several scientific papers, that he also wrote to various journals on the subject (see:List of works by Joseph Priestley
Joseph Priestley (1733–1804) was a British natural philosopher, Dissenting clergyman, political theorist, theologian, and educator. He is best known for his discovery, simultaneously with Antoine Lavoisier, of oxygen gas.
A member of marginal ...
).
Books from 1772–1790:
''Directions for Impregnating Water with Fixed Air''
London, 1772.
''Experiments and Observations on Different Kinds of Air, Vol.1''
London, 1774.
''Experiments and Observations on Different Kinds of Air, Vol.2''
London, 1775. * ''Experiments and Observations on Different Kinds of Air, Vol.3''. London, 1777. * ''Experiments and Observations relating to various Branches of Natural Philosophy, Vol.1''. 'Experiments and Observations on Different Kinds of Air, Vol.4'' London, 1779.
''Experiments and Observations relating to various Branches of Natural Philosophy, Vol.2''
'Experiments and Observations on Different Kinds of Air, Vol.5'' Birmingham, 1781. * ''Experiments Relating to Phlogiston''. London, 1784. * ''Experiments and Observations relating to various Branches of Natural Philosophy, Vol.3''. 'Experiments and Observations on Different Kinds of Air, Vol.6'' Birmingham, 1786.
''Experiments and Observations on Different Kinds of Air, Vol.1–6''
''In 3 volumes, being the former 6 abridged and methodised, with many additions''. Birmingham, 1790. Books from 1791–1803: * ''Experiments on the Generation of Air from Water; to which are prefixed, Experiments relating to the Decomposition of Dephlogisticated and Inflammable Air''. London, 1793.
''Heads of Lectures on a Course of Experimental Philosophy; delivered at the New College in Hackney''
irst 10 of 36 lectures are about Airs London, 1794. * ''Considerations on the Doctrine of Phlogiston and the Decomposition of Water''. Philadelphia, 1796. * ''Experiments and Observations relating to the Analysis of Atmospherical Air; also farther Experiments relating to the Generation of Air from Water.'' [Red before the American Philosophical Society, Feb.5th and 19th in 1796, and printed in their Transactions. To which are added, ''Considerations on the Doctrine of Phlogiston, and the Decomposition of Water'', addressed to Messrs. Berthollet &c]. London, 1796. * ''Considerations on the Doctrine of Phlogiston and the Decomposition of Water, Part II''. Philadelphia, 1797.Schofield, Robert E. ''The Enlightened Joseph Priestley: A Study of His Life and Work from 1773 to 1804''. University Park: Pennsylvania State University Press (2004). . * ''Doctrine of Phlogiston established and that of the Composition of Water refuted''. Northumberland, 1800. * ''Doctrine of Phlogiston established, with Observations on the Conversion of Iron into Steel, in a Letter to Mr. Nicholson''. Printed in 1803.
Notes
Bibliography
*Fruton, Joseph S. ''Methods and Styles in the Development of Chemistry''. Philadelphia: American Philosophical Society, 2002. *Gibbs, F. W. ''Joseph Priestley: Adventurer in Science and Champion of Truth''. London: Thomas Nelson and Sons, 1965. *Jackson, Joe, ''A World on Fire: A Heretic, An Aristocrat And The Race to Discover Oxygen''. New York: Viking, 2005. . *Kramnick, Isaac. "Eighteenth-Century Science and Radical Social Theory: The Case of Joseph Priestley's Scientific Liberalism." ''Journal of British Studies'' 25 (1986): 1–30. *Kuhn, Thomas. ''The Structure of Scientific Revolutions'', third edition. Chicago: University of Chicago Press, 1996. . *Schaffer, Simon. "Priestley Questions: An Historiographic Survey." ''History of Science'' 22.2 (1984): 151–83. *Schofield, Robert E. ''The Enlightenment of Joseph Priestley: A Study of his Life and Work from 1733 to 1773''. University Park: Pennsylvania State University Press, 1997. . *Schofield, Robert E. ''The Enlightened Joseph Priestley: A Study of His Life and Work from 1773 to 1804''. University Park: Pennsylvania State University Press, 2004. . *Thorpe, T.E. ''Joseph Priestley''. London: J. M. Dent, 1906. *Uglow, Jenny. ''The Lunar Men: Five Friends Whose Curiosity Changed the World''. New York: Farrar, Straus and Giroux, 2002. .External links
* archive.org: *full text
(1772) *
full text
(2nd edition, 1774) {{DEFAULTSORT:Experiments And Observations On Different Kinds Of Air 1774 books Science books Books by Joseph Priestley 1774 in science Oxygen