Energy profile (chemistry) on:
[Wikipedia]
[Google]
[Amazon]
In 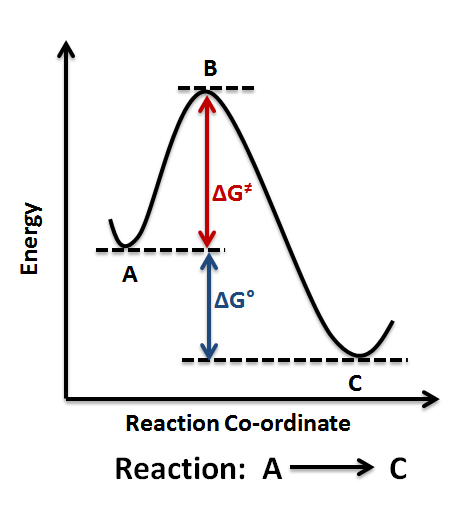 Qualitatively, the reaction coordinate diagrams (one-dimensional energy surfaces) have numerous applications. Chemists use reaction coordinate diagrams as both an analytical and pedagogical aid for rationalizing and illustrating kinetic and
Qualitatively, the reaction coordinate diagrams (one-dimensional energy surfaces) have numerous applications. Chemists use reaction coordinate diagrams as both an analytical and pedagogical aid for rationalizing and illustrating kinetic and
 The concept can be expanded to a tri-atomic molecule such as water where we have two bonds and
The concept can be expanded to a tri-atomic molecule such as water where we have two bonds and 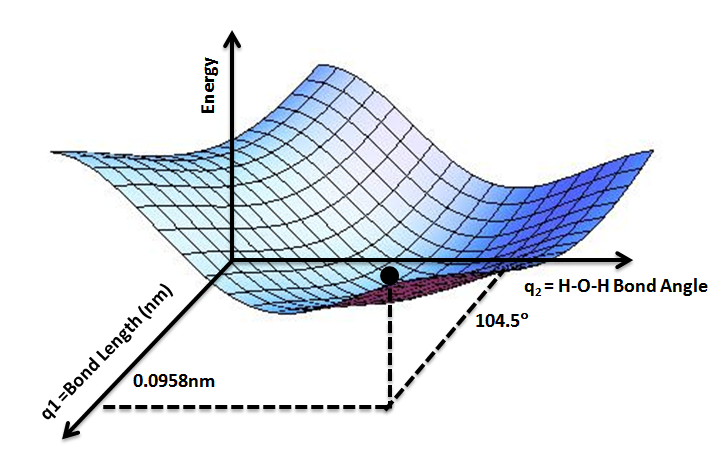 The same concept is applied to organic compounds like
The same concept is applied to organic compounds like

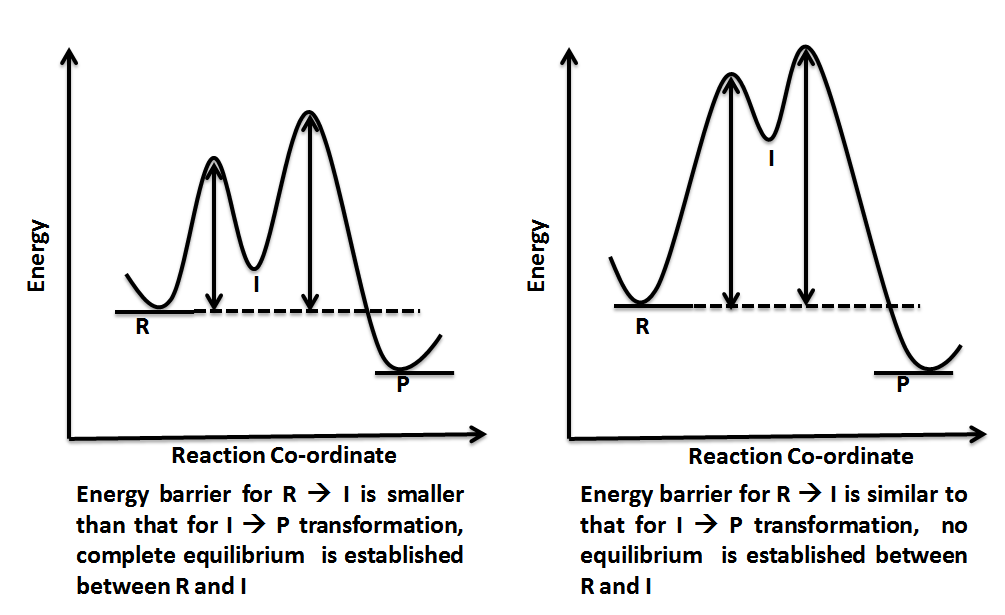
 A reaction involving more than one elementary step has one or more intermediates being formed which, in turn, means there is more than one energy barrier to overcome. In other words, there is more than one transition state lying on the reaction pathway. As it is intuitive that pushing over an energy barrier or passing through a transition state peak would entail the highest energy, it becomes clear that it would be the slowest step in a reaction pathway. However, when more than one such barrier is to be crossed, it becomes important to recognize the highest barrier which will determine the rate of the reaction. This step of the reaction whose rate determines the overall rate of reaction is known as rate determining step or rate limiting step. The height of energy barrier is always measured relative to the energy of the reactant or starting material. Different possibilities have been shown in figure 6.
A reaction involving more than one elementary step has one or more intermediates being formed which, in turn, means there is more than one energy barrier to overcome. In other words, there is more than one transition state lying on the reaction pathway. As it is intuitive that pushing over an energy barrier or passing through a transition state peak would entail the highest energy, it becomes clear that it would be the slowest step in a reaction pathway. However, when more than one such barrier is to be crossed, it becomes important to recognize the highest barrier which will determine the rate of the reaction. This step of the reaction whose rate determines the overall rate of reaction is known as rate determining step or rate limiting step. The height of energy barrier is always measured relative to the energy of the reactant or starting material. Different possibilities have been shown in figure 6.
 Reaction coordinate diagrams also give information about the equilibrium between a reactant or a product and an intermediate. If the barrier energy for going from intermediate to product is much higher than the one for reactant to intermediate transition, it can be safely concluded that a complete equilibrium is established between the reactant and intermediate. However, if the two energy barriers for reactant-to-intermediate and intermediate-to-product transformation are nearly equal, then no complete equilibrium is established and steady state approximation is invoked to derive the kinetic rate expressions for such a reaction.
Reaction coordinate diagrams also give information about the equilibrium between a reactant or a product and an intermediate. If the barrier energy for going from intermediate to product is much higher than the one for reactant to intermediate transition, it can be safely concluded that a complete equilibrium is established between the reactant and intermediate. However, if the two energy barriers for reactant-to-intermediate and intermediate-to-product transformation are nearly equal, then no complete equilibrium is established and steady state approximation is invoked to derive the kinetic rate expressions for such a reaction.
 The relative stability of reactant and product does not define the feasibility of any reaction all by itself. For any reaction to proceed, the starting material must have enough energy to cross over an energy barrier. This energy barrier is known as activation energy (∆''G''≠) and the rate of reaction is dependent on the height of this barrier. A low energy barrier corresponds to a fast reaction and high energy barrier corresponds to a slow reaction.
A reaction is in equilibrium when the rate of forward reaction is equal to the rate of reverse reaction. Such a reaction is said to be reversible. If the starting material and product(s) are in equilibrium then their relative abundance is decided by the difference in free energy between them. In principle, all elementary steps are reversible, but in many cases the equilibrium lies so much towards the product side that the starting material is effectively no longer observable or present in sufficient concentration to have an effect on reactivity. Practically speaking, the reaction is considered to be irreversible.
While most reversible processes will have a reasonably small ''K'' of 103 or less, this is not a hard and fast rule, and a number of chemical processes require reversibility of even very favorable reactions. For instance, the reaction of an carboxylic acid with amines to form a salt takes place with ''K'' of 105–6, and at ordinary temperatures, this process is regarded as irreversible. Yet, with sufficient heating, the reverse reaction takes place to allow formation of the tetrahedral intermediate and, ultimately, amide and water. (For an extreme example requiring reversibility of a step with ''K'' > 1011, see
The relative stability of reactant and product does not define the feasibility of any reaction all by itself. For any reaction to proceed, the starting material must have enough energy to cross over an energy barrier. This energy barrier is known as activation energy (∆''G''≠) and the rate of reaction is dependent on the height of this barrier. A low energy barrier corresponds to a fast reaction and high energy barrier corresponds to a slow reaction.
A reaction is in equilibrium when the rate of forward reaction is equal to the rate of reverse reaction. Such a reaction is said to be reversible. If the starting material and product(s) are in equilibrium then their relative abundance is decided by the difference in free energy between them. In principle, all elementary steps are reversible, but in many cases the equilibrium lies so much towards the product side that the starting material is effectively no longer observable or present in sufficient concentration to have an effect on reactivity. Practically speaking, the reaction is considered to be irreversible.
While most reversible processes will have a reasonably small ''K'' of 103 or less, this is not a hard and fast rule, and a number of chemical processes require reversibility of even very favorable reactions. For instance, the reaction of an carboxylic acid with amines to form a salt takes place with ''K'' of 105–6, and at ordinary temperatures, this process is regarded as irreversible. Yet, with sufficient heating, the reverse reaction takes place to allow formation of the tetrahedral intermediate and, ultimately, amide and water. (For an extreme example requiring reversibility of a step with ''K'' > 1011, see 
 *''SN2:'' For an SN2 mechanism a strongly basic nucleophile (i.e. a charged nucleophile) is favorable. In figure 11 below the rate determining step for
*''SN2:'' For an SN2 mechanism a strongly basic nucleophile (i.e. a charged nucleophile) is favorable. In figure 11 below the rate determining step for  Catalysts: There are two types of
Catalysts: There are two types of 
theoretical chemistry
Theoretical chemistry is the branch of chemistry which develops theoretical generalizations that are part of the theoretical arsenal of modern chemistry: for example, the concepts of chemical bonding, chemical reaction, valence, the surface o ...
, an energy profile is a theoretical representation of a chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breakin ...
or process
A process is a series or set of activities that interact to produce a result; it may occur once-only or be recurrent or periodic.
Things called a process include:
Business and management
* Business process, activities that produce a specific s ...
as a single energetic pathway as the reactant
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
s are transformed into product
Product may refer to:
Business
* Product (business), an item that serves as a solution to a specific consumer problem.
* Product (project management), a deliverable or set of deliverables that contribute to a business solution
Mathematics
* Produ ...
s. This pathway runs along the reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities. In molecu ...
, which is a parametric curve
In mathematics, a parametric equation defines a group of quantities as functions of one or more independent variables called parameters. Parametric equations are commonly used to express the coordinates of the points that make up a geometric ob ...
that follows the pathway of the reaction and indicates its progress; thus, energy profiles are also called reaction coordinate diagrams. They are derived from the corresponding potential energy surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates ...
(PES), which is used in computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into computer programs, to calculate the structures and properties of m ...
to model chemical reactions by relating the energy of a molecule(s) to its structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
(within the Born–Oppenheimer approximation
In quantum chemistry and molecular physics, the Born–Oppenheimer (BO) approximation is the best-known mathematical approximation in molecular dynamics. Specifically, it is the assumption that the wave functions of atomic nuclei and electro ...
).
 Qualitatively, the reaction coordinate diagrams (one-dimensional energy surfaces) have numerous applications. Chemists use reaction coordinate diagrams as both an analytical and pedagogical aid for rationalizing and illustrating kinetic and
Qualitatively, the reaction coordinate diagrams (one-dimensional energy surfaces) have numerous applications. Chemists use reaction coordinate diagrams as both an analytical and pedagogical aid for rationalizing and illustrating kinetic and thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of ther ...
events. The purpose of energy profiles and surfaces is to provide a qualitative representation of how potential energy varies with molecular motion for a given reaction or process./>
Potential energy surfaces
In simplest terms, apotential energy surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates ...
or PES is a mathematical or graphical representation of the relation between energy of a molecule and its geometry. The methods for describing the potential energy are broken down into a classical mechanics interpretation (molecular mechanics
Molecular mechanics uses classical mechanics to model molecular systems. The Born–Oppenheimer approximation is assumed valid and the potential energy of all systems is calculated as a function of the nuclear coordinates using force fields. Mo ...
) and a quantum mechanical
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
interpretation. In the quantum mechanical interpretation an exact expression for energy can be obtained for any molecule derived from quantum principles (although an infinite basis set may be required) but ab initio
''Ab initio'' ( ) is a Latin term meaning "from the beginning" and is derived from the Latin ''ab'' ("from") + ''initio'', ablative singular of ''initium'' ("beginning").
Etymology
Circa 1600, from Latin, literally "from the beginning", from abl ...
calculations/methods will often use approximations to reduce computational cost./>/> Molecular mechanics is empirically based and potential energy is described as a function of component terms that correspond to individual potential functions such as torsion
Torsion may refer to:
Science
* Torsion (mechanics), the twisting of an object due to an applied torque
* Torsion of spacetime, the field used in Einstein–Cartan theory and
** Alternatives to general relativity
* Torsion angle, in chemistry
Bi ...
, stretches, bends, Van der Waals energies, electrostatics and cross terms./> Each component potential function is fit to experimental data or properties predicted by ''ab initio
''Ab initio'' ( ) is a Latin term meaning "from the beginning" and is derived from the Latin ''ab'' ("from") + ''initio'', ablative singular of ''initium'' ("beginning").
Etymology
Circa 1600, from Latin, literally "from the beginning", from abl ...
'' calculations./> Molecular mechanics is useful in predicting equilibrium geometries and transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
s as well as relative conformational stability. As a reaction occurs the atoms of the molecules involved will generally undergo some change in spatial orientation through internal motion as well as its electronic environment. Distortions in the geometric parameters result in a deviation from the equilibrium geometry (local energy minima). These changes in geometry of a molecule or interactions between molecules are dynamic processes which call for understanding all the forces operating within the system. Since these forces can be mathematically derived as first derivative of potential energy with respect to a displacement, it makes sense to map the potential energy of the system as a function of geometric parameters , , and so on./> The potential energy at given values of the geometric parameters is represented as a hyper-surface (when ) or a surface (when ). Mathematically, it can be written as
:
For the quantum mechanical
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
interpretation, a PES is typically defined within the Born–Oppenheimer approximation
In quantum chemistry and molecular physics, the Born–Oppenheimer (BO) approximation is the best-known mathematical approximation in molecular dynamics. Specifically, it is the assumption that the wave functions of atomic nuclei and electro ...
(in order to distinguish between nuclear and electronic motion and energy) which states that the nuclei are stationary relative to the electrons. In other words, the approximation allows the kinetic energy of the nuclei (or movement of the nuclei) to be neglected and therefore the nuclei repulsion is a constant value (as static point charge
A point particle (ideal particle or point-like particle, often spelled pointlike particle) is an idealization of particles heavily used in physics. Its defining feature is that it lacks spatial extension; being dimensionless, it does not take u ...
s) and is only considered when calculating the total energy of the system. The electronic energy is then taken to depend parametrically on the nuclear coordinates, meaning a new electronic energy () must be calculated for each corresponding atomic configuration./>
PES is an important concept in computational chemistry and greatly aids in geometry and transition state optimization.
Degrees of freedom
An -atom system is defined by coordinates: for each atom. Thesedegrees of freedom
Degrees of freedom (often abbreviated df or DOF) refers to the number of independent variables or parameters of a thermodynamic system. In various scientific fields, the word "freedom" is used to describe the limits to which physical movement or ...
can be broken down to include 3 overall translational and 3 (or 2) overall rotational degrees of freedom for a non-linear system (for a linear system). However, overall translational or rotational degrees do not affect the potential energy of the system, which only depends on its internal coordinates. Thus an -atom system will be defined by (non-linear) or (linear) coordinates./> These internal coordinates may be represented by simple stretch, bend, torsion coordinates, or symmetry-adapted linear combinations, or redundant coordinates, or normal modes coordinates, etc. For a system described by -internal coordinates a separate potential energy function can be written with respect to each of these coordinates by holding the other parameters at a constant value allowing the potential energy contribution from a particular molecular motion (or interaction) to be monitored while the other parameters are defined.
Consider a diatomic molecule AB which can macroscopically visualized as two balls (which depict the two atoms A and B) connected through a spring which depicts the bond. As this spring (or bond) is stretched or compressed, the potential energy of the ball-spring system (AB molecule) changes and this can be mapped on a 2-dimensional plot as a function of distance between A and B, i.e. bond length.
 The concept can be expanded to a tri-atomic molecule such as water where we have two bonds and
The concept can be expanded to a tri-atomic molecule such as water where we have two bonds and bond angle
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Chemical bond, the attraction of atoms, ions or molecules to form chemical ...
as variables on which the potential energy of a water molecule will depend. We can safely assume the two bonds to be equal. Thus, a PES can be drawn mapping the potential energy E of a water molecule as a function of two geometric parameters, bond length and bond angle. The lowest point on such a PES will define the equilibrium structure of a water molecule.
 The same concept is applied to organic compounds like
The same concept is applied to organic compounds like ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petro ...
, butane
Butane () or ''n''-butane is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature. The name bu ...
etc. to define their lowest energy and most stable conformations.
Characterizing a PES
The most important points on a PES are the stationary points where the surface is flat, i.e. parallel to a horizontal line corresponding to one geometric parameter, a plane corresponding to two such parameters or even a hyper-plane corresponding to more than two geometric parameters. The energy values corresponding to the transition states and the ground state of the reactants and products can be found using the potential energy function by calculating the function's critical points or the stationary points. Stationary points occur when the 1stpartial derivative
In mathematics, a partial derivative of a function of several variables is its derivative with respect to one of those variables, with the others held constant (as opposed to the total derivative, in which all variables are allowed to vary). Par ...
of the energy with respect to each geometric parameter is equal to zero.
:
Using analytical derivatives of the derived expression for energy, one can find and characterize a stationary point as minimum, maximum or a saddle point
In mathematics, a saddle point or minimax point is a point on the surface of the graph of a function where the slopes (derivatives) in orthogonal directions are all zero (a critical point), but which is not a local extremum of the function. ...
. The ground states are represented by local energy minima and the transition states by saddle points.
Minima represent stable or quasi-stable species, i.e. reactants and products with finite lifetime. Mathematically, a minimum point is given as
:
:
A point may be local minimum when it is lower in energy compared to its surrounding only or a global minimum which is the lowest energy point on the entire potential energy surface.
Saddle point represents a maximum along only one direction (that of the reaction coordinate) and is a minimum along all other directions. In other words, a saddle point represents a transition state along the reaction coordinate. Mathematically, a saddle point occurs when
:
for all except along the reaction coordinate and
:
along the reaction coordinate.

Reaction coordinate diagrams
The intrinsic reaction coordinate (IRC), derived from the potential energy surface, is a parametric curve that connects two energy minima in the direction that traverses the minimum energy barrier (or shallowest ascent) passing through one or more saddle point(s). However, in reality if reacting species attains enough energy it may deviate from the IRC to some extent./> The energy values (points on the hyper-surface) along the reaction coordinate result in a 1-D energy surface (a line) and when plotted against the reaction coordinate (energy vs reaction coordinate) gives what is called a reaction coordinate diagram (or energy profile). Another way of visualizing an energy profile is as a cross section of the hyper surface, or surface, long the reaction coordinate. Figure 5 shows an example of a cross section, represented by the plane, taken along the reaction coordinate and the potential energy is represented as a function or composite of two geometric variables to form a 2-D energy surface. In principle, the potential energy function can depend on N variables but since an accurate visual representation of a function of 3 or more variables cannot be produced (excluding level hypersurfaces) a 2-D surface has been shown. The points on the surface that intersect the plane are then projected onto the reaction coordinate diagram (shown on the right) to produce a 1-D slice of the surface along the IRC. The reaction coordinate is described by its parameters, which are frequently given as a composite of several geometric parameters, and can change direction as the reaction progresses so long as the smallest energy barrier (or activation energy (Ea)) is traversed./> The saddle point represents the highest energy point lying on the reaction coordinate connecting the reactant and product; this is known as the transition state. A reaction coordinate diagram may also have one or more transient intermediates which are shown by high energy wells connected via a transition state peak. Any chemical structure that lasts longer than the time for typical bond vibrations (10−13 – 10−14s) can be considered as intermediate./> A reaction involving more than one elementary step has one or more intermediates being formed which, in turn, means there is more than one energy barrier to overcome. In other words, there is more than one transition state lying on the reaction pathway. As it is intuitive that pushing over an energy barrier or passing through a transition state peak would entail the highest energy, it becomes clear that it would be the slowest step in a reaction pathway. However, when more than one such barrier is to be crossed, it becomes important to recognize the highest barrier which will determine the rate of the reaction. This step of the reaction whose rate determines the overall rate of reaction is known as rate determining step or rate limiting step. The height of energy barrier is always measured relative to the energy of the reactant or starting material. Different possibilities have been shown in figure 6.
A reaction involving more than one elementary step has one or more intermediates being formed which, in turn, means there is more than one energy barrier to overcome. In other words, there is more than one transition state lying on the reaction pathway. As it is intuitive that pushing over an energy barrier or passing through a transition state peak would entail the highest energy, it becomes clear that it would be the slowest step in a reaction pathway. However, when more than one such barrier is to be crossed, it becomes important to recognize the highest barrier which will determine the rate of the reaction. This step of the reaction whose rate determines the overall rate of reaction is known as rate determining step or rate limiting step. The height of energy barrier is always measured relative to the energy of the reactant or starting material. Different possibilities have been shown in figure 6.
 Reaction coordinate diagrams also give information about the equilibrium between a reactant or a product and an intermediate. If the barrier energy for going from intermediate to product is much higher than the one for reactant to intermediate transition, it can be safely concluded that a complete equilibrium is established between the reactant and intermediate. However, if the two energy barriers for reactant-to-intermediate and intermediate-to-product transformation are nearly equal, then no complete equilibrium is established and steady state approximation is invoked to derive the kinetic rate expressions for such a reaction.
Reaction coordinate diagrams also give information about the equilibrium between a reactant or a product and an intermediate. If the barrier energy for going from intermediate to product is much higher than the one for reactant to intermediate transition, it can be safely concluded that a complete equilibrium is established between the reactant and intermediate. However, if the two energy barriers for reactant-to-intermediate and intermediate-to-product transformation are nearly equal, then no complete equilibrium is established and steady state approximation is invoked to derive the kinetic rate expressions for such a reaction.

Drawing a reaction coordinate diagram
Although a reaction coordinate diagram is essentially derived from a potential energy surface, it is not always feasible to draw one from a PES. A chemist draws a reaction coordinate diagram for a reaction based on the knowledge of free energy or enthalpy change associated with the transformation which helps him to place the reactant and product into perspective and whether any intermediate is formed or not. One guideline for drawing diagrams for complex reactions is the principle of least motion which says that a favored reaction proceeding from a reactant to an intermediate or from one intermediate to another or product is one which has the least change in nuclear position or electronic configuration. Thus, it can be said that the reactions involving dramatic changes in position of nuclei actually occur through a series of simple chemical reactions. Hammond postulate is another tool which assists in drawing the energy of a transition state relative to a reactant, an intermediate or a product. It states that the transition state resembles the reactant, intermediate or product that it is closest in energy to, as long the energy difference between the transition state and the adjacent structure is not too large. This postulate helps to accurately predict the shape of a reaction coordinate diagram and also gives an insight into the molecular structure at the transition state.Kinetic and thermodynamic considerations
A chemical reaction can be defined by two important parameters- theGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and p ...
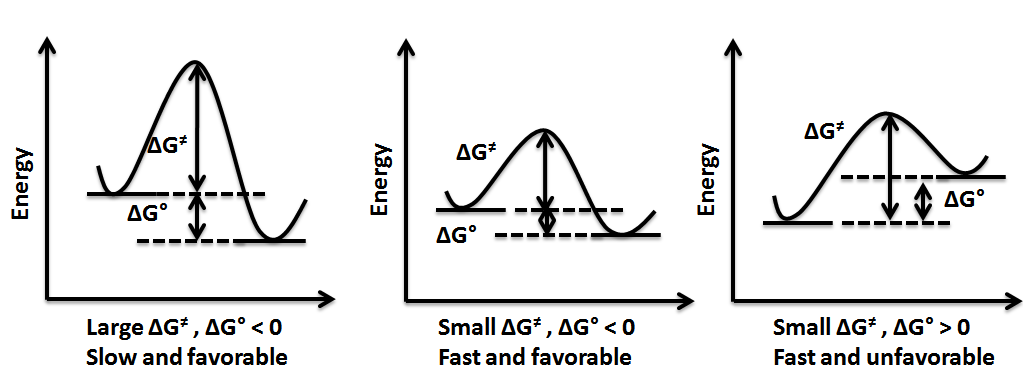
associated with a chemical transformation and the rate of such a transformation. These parameters are independent of each other. While free energy change describes the stability of products relative to reactants, the rate of any reaction is defined by the energy of the transition state relative to the starting material. Depending on these parameters, a reaction can be favorable or unfavorable, fast or slow and reversible or irreversible, as shown in figure 8.
A favorable reaction is one in which the change in free energy ∆''G''° is negative (exergonic
An exergonic process is one which there is a positive flow of energy from the system to the surroundings. This is in contrast with an endergonic process. Constant pressure, constant temperature reactions are exergonic if and only if the Gibbs f ...
) or in other words, the free energy of product, ''G''°product, is less than the free energy of the starting materials, ''G''°reactant. ∆''G''°> 0 (endergonic
In chemical thermodynamics, an endergonic reaction (; also called a heat absorbing nonspontaneous reaction or an unfavorable reaction) is a chemical reaction in which the standard change in free energy is positive, and an additional driving fo ...
) corresponds to an unfavorable reaction. The ∆''G''° can be written as a function of change in enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant p ...
(∆''H''°) and change in entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
(∆''S''°) as ∆''G''°= ∆''H''° – ''T''∆''S''°. Practically, enthalpies, not free energy, are used to determine whether a reaction is favorable or unfavorable, because ∆''H''° is easier to measure and ''T''∆''S''° is usually too small to be of any significance (for ''T'' < 100 °C). A reaction with ∆''H''°<0 is called exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity ( ...
reaction while one with ∆''H''°>0 is endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. p. ...
.
 The relative stability of reactant and product does not define the feasibility of any reaction all by itself. For any reaction to proceed, the starting material must have enough energy to cross over an energy barrier. This energy barrier is known as activation energy (∆''G''≠) and the rate of reaction is dependent on the height of this barrier. A low energy barrier corresponds to a fast reaction and high energy barrier corresponds to a slow reaction.
A reaction is in equilibrium when the rate of forward reaction is equal to the rate of reverse reaction. Such a reaction is said to be reversible. If the starting material and product(s) are in equilibrium then their relative abundance is decided by the difference in free energy between them. In principle, all elementary steps are reversible, but in many cases the equilibrium lies so much towards the product side that the starting material is effectively no longer observable or present in sufficient concentration to have an effect on reactivity. Practically speaking, the reaction is considered to be irreversible.
While most reversible processes will have a reasonably small ''K'' of 103 or less, this is not a hard and fast rule, and a number of chemical processes require reversibility of even very favorable reactions. For instance, the reaction of an carboxylic acid with amines to form a salt takes place with ''K'' of 105–6, and at ordinary temperatures, this process is regarded as irreversible. Yet, with sufficient heating, the reverse reaction takes place to allow formation of the tetrahedral intermediate and, ultimately, amide and water. (For an extreme example requiring reversibility of a step with ''K'' > 1011, see
The relative stability of reactant and product does not define the feasibility of any reaction all by itself. For any reaction to proceed, the starting material must have enough energy to cross over an energy barrier. This energy barrier is known as activation energy (∆''G''≠) and the rate of reaction is dependent on the height of this barrier. A low energy barrier corresponds to a fast reaction and high energy barrier corresponds to a slow reaction.
A reaction is in equilibrium when the rate of forward reaction is equal to the rate of reverse reaction. Such a reaction is said to be reversible. If the starting material and product(s) are in equilibrium then their relative abundance is decided by the difference in free energy between them. In principle, all elementary steps are reversible, but in many cases the equilibrium lies so much towards the product side that the starting material is effectively no longer observable or present in sufficient concentration to have an effect on reactivity. Practically speaking, the reaction is considered to be irreversible.
While most reversible processes will have a reasonably small ''K'' of 103 or less, this is not a hard and fast rule, and a number of chemical processes require reversibility of even very favorable reactions. For instance, the reaction of an carboxylic acid with amines to form a salt takes place with ''K'' of 105–6, and at ordinary temperatures, this process is regarded as irreversible. Yet, with sufficient heating, the reverse reaction takes place to allow formation of the tetrahedral intermediate and, ultimately, amide and water. (For an extreme example requiring reversibility of a step with ''K'' > 1011, see demethylation Demethylation is the chemical process resulting in the removal of a methyl group (CH3) from a molecule. A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen ato ...
.) A reaction can also be rendered irreversible if a subsequent, faster step takes place to consume the initial product(s), or a gas is evolved in an open system. Thus, there is no value of ''K'' that serves as a "dividing line" between reversible and irreversible processes. Instead, reversibility depends on timescale, temperature, the reaction conditions, and the overall energy landscape.
When a reactant can form two different products depending on the reaction conditions, it becomes important to choose the right conditions to favor the desired product. If a reaction is carried out at relatively lower temperature, then the product formed is one lying across the smaller energy barrier. This is called kinetic control and the ratio of the products formed depends on the relative energy barriers leading to the products. Relative stabilities of the products do not matter. However, at higher temperatures the molecules have enough energy to cross over both energy barriers leading to the products. In such a case, the product ratio is determined solely by the energies of the products and energies of the barrier do not matter. This is known as thermodynamic control and it can only be achieved when the products can inter-convert and equilibrate under the reaction condition. A reaction coordinate diagram can also be used to qualitatively illustrate kinetic and thermodynamic control in a reaction.

Applications
Following are few examples on how to interpret reaction coordinate diagrams and use them in analyzing reactions. Solvent Effect: In general, if the transition state for the rate determining step corresponds to a more charged species relative to the starting material then increasing the polarity of the solvent will increase the rate of the reaction since a more polar solvent be more effective at stabilizing the transition state (ΔG‡ would decrease). If the transition state structure corresponds to a less charged species then increasing the solvents polarity would decrease the reaction rate since a more polar solvent would be more effective at stabilizing the starting material (ΔGo would decrease which in turn increases ΔG‡). ''SN1 vs SN2'' The SN1 and SN2 mechanisms are used as an example to demonstrate how solvent effects can be indicated in reaction coordinate diagrams. *''SN1:'' Figure 10 shows the rate determining step for an SN1 mechanism, formation of thecarbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encounte ...
intermediate, and the corresponding reaction coordinate diagram. For an SN1 mechanism the transition state structure shows a partial charge density relative to the neutral ground state structure. Therefore, increasing the solvent polarity, for example from hexanes (shown as blue) to ether (shown in red), would decrease the rate of the reaction. As shown in figure 9, the starting material has approximately the same stability in both solvents (therefore ΔΔGo=ΔGopolar - ΔGonon polar is small) and the transition state is stabilized more in ether meaning ΔΔG≠ = ΔG≠polar - ΔG≠non-polar is large.
 *''SN2:'' For an SN2 mechanism a strongly basic nucleophile (i.e. a charged nucleophile) is favorable. In figure 11 below the rate determining step for
*''SN2:'' For an SN2 mechanism a strongly basic nucleophile (i.e. a charged nucleophile) is favorable. In figure 11 below the rate determining step for Williamson ether synthesis
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide io ...
is shown. The starting material is methyl chloride and an ethoxide ion which has a ''localized'' negative charge meaning it is more stable in polar solvents. The figure shows a transition state structure as the methyl chloride undergoes nucleophilic attack. In the transition state structure the charge is distributed between the Cl and the O atoms and the more polar solvent is less effective at stabilizing the transition state structure relative to the starting materials. In other words, the energy difference between the polar and non-polar solvent is greater for the ground state (for the starting material) than in the transition state.
 Catalysts: There are two types of
Catalysts: There are two types of catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, positive and negative. Positive catalysts increase the reaction rate and negative catalysts (or inhibitors) slow down a reaction and possibly cause the reaction not occur at all. The purpose of a catalyst is to alter the activation energy. Figure 12 illustrates the purpose of a catalyst in that only the activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
is changed and not the relative thermodynamic stabilities, shown in the figure as ΔH, of the products and reactants. This means that a catalyst will not alter the equilibrium concentrations of the products and reactants but will only allow the reaction to reach equilibrium faster. Figure 13 shows the catalyzed pathway occurring in multiple steps which is a more realistic depiction of a catalyzed process. The new catalyzed pathway can occur through the same mechanism as the uncatalyzed reaction or through an alternate mechanism./> An enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. ...
is a biological catalyst that increases the rate for many vital biochemical reactions. Figure 13 shows a common way to illustrate the effect of an enzyme on a given biochemical reaction.
See also
*Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and p ...
*Enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant p ...
*Entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
*Computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into computer programs, to calculate the structures and properties of m ...
*Molecular mechanics
Molecular mechanics uses classical mechanics to model molecular systems. The Born–Oppenheimer approximation is assumed valid and the potential energy of all systems is calculated as a function of the nuclear coordinates using force fields. Mo ...
*Born–Oppenheimer approximation
In quantum chemistry and molecular physics, the Born–Oppenheimer (BO) approximation is the best-known mathematical approximation in molecular dynamics. Specifically, it is the assumption that the wave functions of atomic nuclei and electro ...
References
{{Reaction mechanisms Computational chemistry