Ergocryptine on:
[Wikipedia]
[Google]
[Amazon]
Ergocryptine is an
 The next step in the biosynthesis of ergocryptine is the transformation of 4-dimethylallyl abrine to Chanoclavine-I. It has been shown that the [enzyme EasE and EasC (FgaOx1 and FgaCat in A. fumigatus, respectively) are both required to generate Chanoclavine-I from 4-DMA abrine. Mutation experiments altering these enzymes independently stopped the pathway at abrine. This indicates that cooperation between EasE and EasC is necessary.
The next step in the biosynthesis of ergocryptine is the transformation of 4-dimethylallyl abrine to Chanoclavine-I. It has been shown that the [enzyme EasE and EasC (FgaOx1 and FgaCat in A. fumigatus, respectively) are both required to generate Chanoclavine-I from 4-DMA abrine. Mutation experiments altering these enzymes independently stopped the pathway at abrine. This indicates that cooperation between EasE and EasC is necessary.

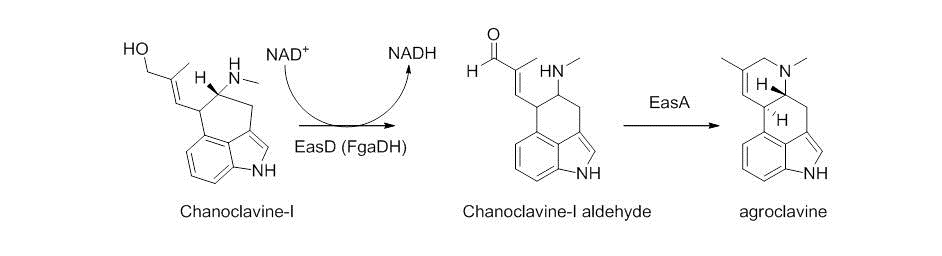 Chanocalvine-I is then oxidized to chanoclavine-I aldehyde with NAD+ dependent enzyme EasD (FgaDH in A. fumigatus). Chanoclavine-I aldehyde is a branch point, leading to different ergot alkaloids, depending on the specific fungus. In C. purpurea, chanoclavine-I aldehyde is converted to argoclavine with EasA, referred to as the old yellow enzyme or FgaOx3. This process occurs via keto-enol tautomerization to facilitate rotation about a carbon-carbon bond, followed by tautomerization back to the aldehyde, and condensation with the proximal secondary amine. The iminium species created by cyclization is then reduced to the tertiary amine, yielding agroclavine.
Chanocalvine-I is then oxidized to chanoclavine-I aldehyde with NAD+ dependent enzyme EasD (FgaDH in A. fumigatus). Chanoclavine-I aldehyde is a branch point, leading to different ergot alkaloids, depending on the specific fungus. In C. purpurea, chanoclavine-I aldehyde is converted to argoclavine with EasA, referred to as the old yellow enzyme or FgaOx3. This process occurs via keto-enol tautomerization to facilitate rotation about a carbon-carbon bond, followed by tautomerization back to the aldehyde, and condensation with the proximal secondary amine. The iminium species created by cyclization is then reduced to the tertiary amine, yielding agroclavine.
 A cytochrome P-450 monooxygenase enzyme catalyzes a two electron oxidation of agroclavne to the corresponding primary alcohol, elymoclavine. Elymoclavine is then oxidized by four electrons by a P450 monooxygenase to give paspalic acid. Paspalic acid then undergoes isomerization of the carbon-carbon double bond that is in conjugation with the acid, to give D-lysergic acid.
A cytochrome P-450 monooxygenase enzyme catalyzes a two electron oxidation of agroclavne to the corresponding primary alcohol, elymoclavine. Elymoclavine is then oxidized by four electrons by a P450 monooxygenase to give paspalic acid. Paspalic acid then undergoes isomerization of the carbon-carbon double bond that is in conjugation with the acid, to give D-lysergic acid.
 Lysergic Acid is a branch point in the biosynthesis of ergoamides and ergopeptines. On the path to ergocryptine, an ergopeptine, the tripeptide is installed by a Non-Ribosomal Peptide Synthase (NRPS). It has been shown that there are two enzymes, D-lysergyl peptide synthases (LPS) 1 and 2, which are responsible for the tripeptide connection to lysergic acid. The timing of the oxidation of valine to an alcohol is not exactly known. However, it is speculated that the oxidation occurs while bound to the NRPS LPS2. Ergocryptine is found in two forms, differing in the amino acid used by the NRPS. The alpha form contains the amino acid leucine, while the beta-form uses the amino acid isoleucine.
Lysergic Acid is a branch point in the biosynthesis of ergoamides and ergopeptines. On the path to ergocryptine, an ergopeptine, the tripeptide is installed by a Non-Ribosomal Peptide Synthase (NRPS). It has been shown that there are two enzymes, D-lysergyl peptide synthases (LPS) 1 and 2, which are responsible for the tripeptide connection to lysergic acid. The timing of the oxidation of valine to an alcohol is not exactly known. However, it is speculated that the oxidation occurs while bound to the NRPS LPS2. Ergocryptine is found in two forms, differing in the amino acid used by the NRPS. The alpha form contains the amino acid leucine, while the beta-form uses the amino acid isoleucine.

ergopeptine
Ergoline is a core structure in many alkaloids and their synthetic derivatives. Ergoline alkaloids were first characterized in ergot. Some of these are implicated in the condition of ergotism, which can take a convulsive form or a gangrenous for ...
and one of the ergoline
Ergoline is a core structure in many alkaloids and their synthetic derivatives. Ergoline alkaloids were first characterized in ergot. Some of these are implicated in the condition of ergotism, which can take a convulsive form or a gangrenous for ...
alkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large varie ...
s. It is isolated from ergot
Ergot ( ) or ergot fungi refers to a group of fungi of the genus ''Claviceps''.
The most prominent member of this group is '' Claviceps purpurea'' ("rye ergot fungus"). This fungus grows on rye and related plants, and produces alkaloids that c ...
or fermentation broth and it serves as starting material for the production of bromocriptine
Bromocriptine, originally marketed as Parlodel and subsequently under many brand names, is an ergoline derivative and dopamine agonist that is used in the treatment of pituitary tumors, Parkinson's disease, hyperprolactinaemia, neuroleptic malig ...
. Two isomers of ergocryptine exist, α-ergocryptine and β-ergocryptine. The ''beta'' differs from the ''alpha'' form only in the position of a single methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as ...
group, which is a consequence of the biosynthesis
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-Catalysis, catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthe ...
in which the proteinogenic amino acid
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation from RNA. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) ...
leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-Car ...
is replaced by isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the depro ...
. β-Ergocryptine was first identified in 1967 by Albert Hofmann
Albert Hofmann (11 January 1906 – 29 April 2008) was a Swiss chemist known for being the first to synthesize, ingest, and learn of the psychedelic effects of lysergic acid diethylamide (LSD). Hofmann's team also isolated, named and synthesi ...
. Ergot from different sources have different ratios of the two isomers.
Biosynthesis
Thebiosynthetic
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme- catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthesis) serve ...
pathways to ergocryptine starts with the prenylation of L-tryptophan in an SN1 fashion with dimethylallyl pyrophosphate
Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynt ...
(DMAPP). DMAPP is derived from mevalonic acid
Mevalonic acid (MVA) is a key organic compound in biochemistry; the name is a contraction of dihydroxymethylvalerolactone. The carboxylate anion of mevalonic acid, which is the predominant form in biological environments, is known as ''mevalonat ...
. This reaction is catalyzed by a prenyltransferase
Prenyltransferases (PTs) are a class of enzymes that transfer allylic prenyl groups to acceptor molecules. Prenyl transferases commonly refer to isoprenyl diphosphate syntheses (IPPSs). Prenyltransferases are a functional category and include seve ...
enzyme (Prenyltransferase 4-dimethylallyltryptophan synthase) named FgaPT2 in Aspergillus fumigatus
''Aspergillus fumigatus'' is a species of fungus in the genus ''Aspergillus'', and is one of the most common ''Aspergillus'' species to cause disease in individuals with an immunodeficiency.
''Aspergillus fumigatus'', a saprotroph widespread in ...
. An X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
structure of the prenyltransferase FgaPT2 and tryptophan has been reported, and used to propose a three step mechanism: (1) formation of allylic carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
; (2) nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
attack of tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromat ...
on the carbocation; (3) deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
to restore aromaticity and generate the product, 4-dimethylallyltryptophan (DMAT). DMAT is then N-methylated at the amino of the tryptophan backbone with the EasF enzyme, named FgaMT in A. fumigatus. S-adenosylmethionine (SAM) being the methyl source.
 The next step in the biosynthesis of ergocryptine is the transformation of 4-dimethylallyl abrine to Chanoclavine-I. It has been shown that the [enzyme EasE and EasC (FgaOx1 and FgaCat in A. fumigatus, respectively) are both required to generate Chanoclavine-I from 4-DMA abrine. Mutation experiments altering these enzymes independently stopped the pathway at abrine. This indicates that cooperation between EasE and EasC is necessary.
The next step in the biosynthesis of ergocryptine is the transformation of 4-dimethylallyl abrine to Chanoclavine-I. It has been shown that the [enzyme EasE and EasC (FgaOx1 and FgaCat in A. fumigatus, respectively) are both required to generate Chanoclavine-I from 4-DMA abrine. Mutation experiments altering these enzymes independently stopped the pathway at abrine. This indicates that cooperation between EasE and EasC is necessary.

 Chanocalvine-I is then oxidized to chanoclavine-I aldehyde with NAD+ dependent enzyme EasD (FgaDH in A. fumigatus). Chanoclavine-I aldehyde is a branch point, leading to different ergot alkaloids, depending on the specific fungus. In C. purpurea, chanoclavine-I aldehyde is converted to argoclavine with EasA, referred to as the old yellow enzyme or FgaOx3. This process occurs via keto-enol tautomerization to facilitate rotation about a carbon-carbon bond, followed by tautomerization back to the aldehyde, and condensation with the proximal secondary amine. The iminium species created by cyclization is then reduced to the tertiary amine, yielding agroclavine.
Chanocalvine-I is then oxidized to chanoclavine-I aldehyde with NAD+ dependent enzyme EasD (FgaDH in A. fumigatus). Chanoclavine-I aldehyde is a branch point, leading to different ergot alkaloids, depending on the specific fungus. In C. purpurea, chanoclavine-I aldehyde is converted to argoclavine with EasA, referred to as the old yellow enzyme or FgaOx3. This process occurs via keto-enol tautomerization to facilitate rotation about a carbon-carbon bond, followed by tautomerization back to the aldehyde, and condensation with the proximal secondary amine. The iminium species created by cyclization is then reduced to the tertiary amine, yielding agroclavine.
 A cytochrome P-450 monooxygenase enzyme catalyzes a two electron oxidation of agroclavne to the corresponding primary alcohol, elymoclavine. Elymoclavine is then oxidized by four electrons by a P450 monooxygenase to give paspalic acid. Paspalic acid then undergoes isomerization of the carbon-carbon double bond that is in conjugation with the acid, to give D-lysergic acid.
A cytochrome P-450 monooxygenase enzyme catalyzes a two electron oxidation of agroclavne to the corresponding primary alcohol, elymoclavine. Elymoclavine is then oxidized by four electrons by a P450 monooxygenase to give paspalic acid. Paspalic acid then undergoes isomerization of the carbon-carbon double bond that is in conjugation with the acid, to give D-lysergic acid.
 Lysergic Acid is a branch point in the biosynthesis of ergoamides and ergopeptines. On the path to ergocryptine, an ergopeptine, the tripeptide is installed by a Non-Ribosomal Peptide Synthase (NRPS). It has been shown that there are two enzymes, D-lysergyl peptide synthases (LPS) 1 and 2, which are responsible for the tripeptide connection to lysergic acid. The timing of the oxidation of valine to an alcohol is not exactly known. However, it is speculated that the oxidation occurs while bound to the NRPS LPS2. Ergocryptine is found in two forms, differing in the amino acid used by the NRPS. The alpha form contains the amino acid leucine, while the beta-form uses the amino acid isoleucine.
Lysergic Acid is a branch point in the biosynthesis of ergoamides and ergopeptines. On the path to ergocryptine, an ergopeptine, the tripeptide is installed by a Non-Ribosomal Peptide Synthase (NRPS). It has been shown that there are two enzymes, D-lysergyl peptide synthases (LPS) 1 and 2, which are responsible for the tripeptide connection to lysergic acid. The timing of the oxidation of valine to an alcohol is not exactly known. However, it is speculated that the oxidation occurs while bound to the NRPS LPS2. Ergocryptine is found in two forms, differing in the amino acid used by the NRPS. The alpha form contains the amino acid leucine, while the beta-form uses the amino acid isoleucine.

See also
* DihydroergocryptineReferences
{{Ergolines Ergopeptines Ergot alkaloids Lactams Oxazolopyrrolopyrazines Indolizidines Isopropyl compounds Carboxamides