Endothermic Gas on:
[Wikipedia]
[Google]
[Amazon]
Endothermic
An endothermic process is a chemical or physical process that absorbs heat from its surroundings. In terms of thermodynamics, it is a thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, ...
gas is a gas that inhibits or reverses oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
on the surfaces it is in contact with. This gas is the product of incomplete combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
in a controlled environment. An example mixture is hydrogen gas
Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest and most abundant chemical element in the universe, constituting about 75% of all normal matter. Under standard conditions, hydrogen is a gas of diatomi ...
(H2), nitrogen gas
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh i ...
(N2), and carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
(CO). The hydrogen and carbon monoxide are reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
s, so they work together to shield surfaces from oxidation.
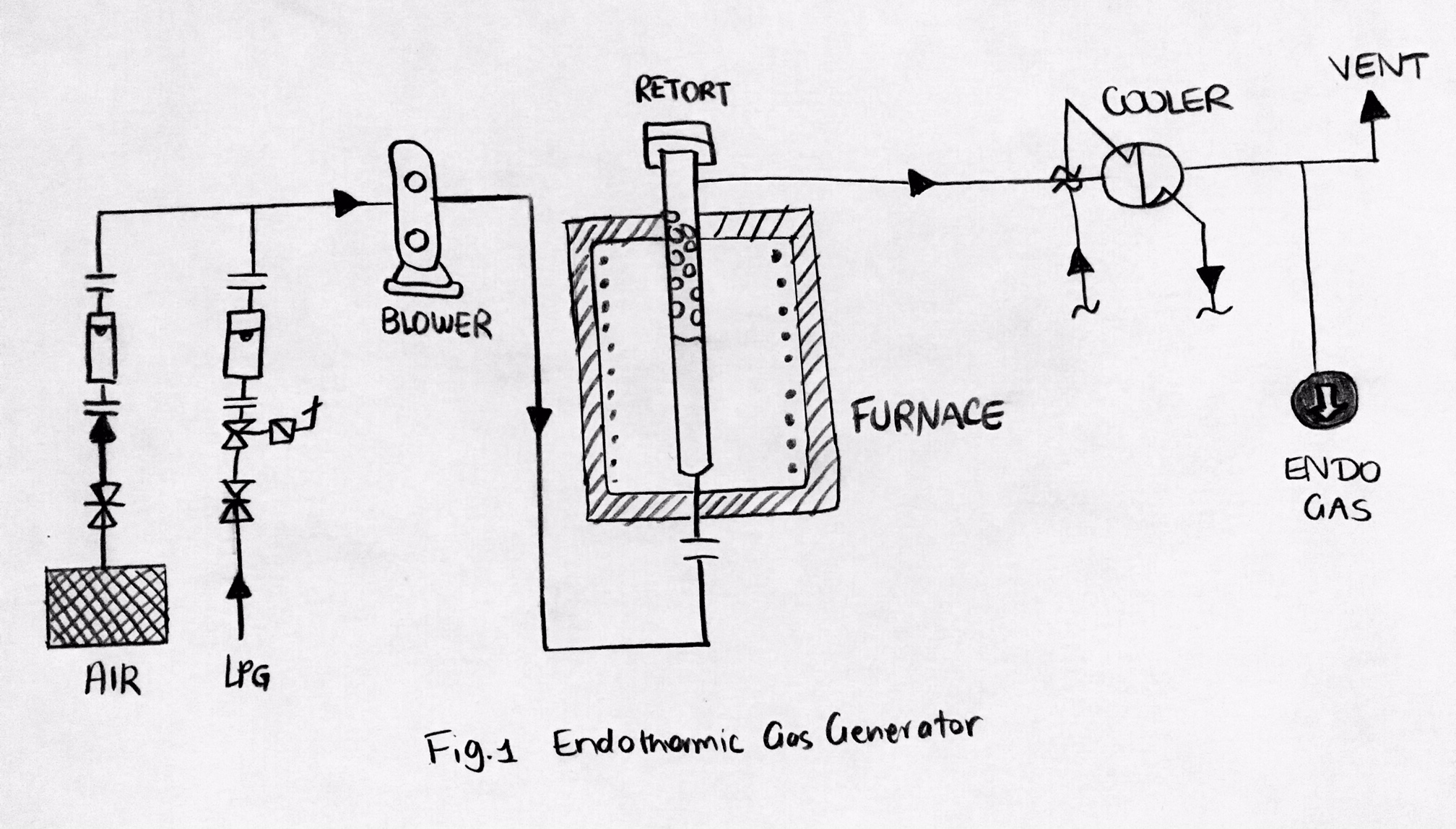
Endothermic gas is often used as a carrier gas for gas carburizing and carbonitriding. An endothermic gas generator could be used to supply heat to form an endothermic reaction.
Synthesised in the catalytic retort(s) of endothermic generators, the gas in the endothermic atmosphere is combined with an additive gas including natural gas
Natural gas (also fossil gas, methane gas, and gas) is a naturally occurring compound of gaseous hydrocarbons, primarily methane (95%), small amounts of higher alkanes, and traces of carbon dioxide and nitrogen, hydrogen sulfide and helium ...
, propane
Propane () is a three-carbon chain alkane with the molecular formula . It is a gas at standard temperature and pressure, but becomes liquid when compressed for transportation and storage. A by-product of natural gas processing and petroleum ref ...
(C3H8) or air and is then used to improve the surface chemistry work positioned in the furnace.

Purposes
There are two common purposes of the atmospheres in the heat treating industry: # Protect the processed material from surface reactions (chemically inert) # Allow surface of processed material to change (chemically reactive)Principal components of a endothermic gas generator
Principal components of endothermic gas generators: # ''Heating chamber'' for supplying heat by electric heating elements ofcombustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
,
# Vertical ''cylindrical retorts'',
# Tiny, porous ''ceramic'' pieces that are saturated with nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
, which acts as a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
for the reaction,
# ''Cooling heat exchanger'' in order to cool the products of the reaction as quickly as possible so that it reaches a particular temperature which stops any further reaction,
# ''Control system'' which will help maintain the consistency of the temperature of the reaction which will help adjust the gas ratio, providing the wanted dew point.
Chemical composition
Chemistry of endothermic gas generators: * N2 (nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
) → 45.1% (volume)
* CO (carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
) → 19.6% (volume)
* CO2 (carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
) → 0.4% (volume)
* H2 (hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
) → 34.6% (volume)
* CH4 (methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
) → 0.3% (volume)
* Dew point
The dew point is the temperature the air needs to be cooled to (at constant pressure) in order to produce a relative humidity of 100%. This temperature depends on the pressure and water content of the air. When the air at a temperature above the ...
→ +20/+50
* Gas ratio → 2.6:1
Applications
Applications of endothermic gas generators: # Annealing: iron and steel #Brazing
Brazing is a metal-joining process in which two or more metal items are joined by melting and flowing a filler metal into the joint, with the filler metal having a lower melting point than the adjoining metal.
Brazing differs from welding in ...
: copper and silver
# Carbon restoration: carburizing, carbonitriding, nitrocarburizing
# Neutral hardening: low, medium and high alloy carbon steels
# Normalizing: iron and steel
# Sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plas ...
: powder metals
# Austempering: ductile iron
It is relatively simple to operate and maintain endothermic gas generators, however, maintenance such as the burnout process is often overlooked.{{cite web, last1=Pye, first1=David, title=Heat Treating Process, url=https://www.asminternational.org/documents/10192/1914052/htp00207p037.pdf/d711c8f7-b88a-4dd2-965a-24ed4b9b474a/HTP00207P037, accessdate=19 June 2018, archive-date=19 July 2018, archive-url=https://web.archive.org/web/20180719234055/https://www.asminternational.org/documents/10192/1914052/htp00207p037.pdf/d711c8f7-b88a-4dd2-965a-24ed4b9b474a/HTP00207P037, url-status=dead
See also
* Forming gasReferences