Elimination Reaction on:
[Wikipedia]
[Google]
[Amazon]
An elimination reaction is a type of
 An example of this type of reaction in ''scheme 1'' is the reaction of
An example of this type of reaction in ''scheme 1'' is the reaction of
 :Only reaction product A results from antiperiplanar elimination. The presence of product B is an indication that an E1 mechanism is occurring.
* It is accompanied by carbocationic
:Only reaction product A results from antiperiplanar elimination. The presence of product B is an indication that an E1 mechanism is occurring.
* It is accompanied by carbocationic
 The next most common type of elimination reaction is α-elimination. For a carbon center, the result of α-elimination is the formation of a carbene, which includes "stable carbenes" such as
The next most common type of elimination reaction is α-elimination. For a carbon center, the result of α-elimination is the formation of a carbene, which includes "stable carbenes" such as
organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organ ...
in which two substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
s are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
, a third type of reaction, E1CB, exists. Finally, the pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
of xanthate
A xanthate is a Salt (chemistry), salt or ester of a xanthic acid. The formula of the salt of xanthic acid is (where R is organyl group and M is usually Sodium, Na or Potassium, K). Xanthate also refers to the anion . The formula of a xanthic a ...
and acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
esters
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ...
proceed through an "internal" elimination mechanism, the Ei mechanism.
E2 mechanism
The E2 mechanism, where E2 stands for bimolecular elimination, involves a one-step mechanism in which ''carbon-hydrogen'' and ''carbon-halogen'' bonds break to form a double bond (''C=CPi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
'').
The specifics of the reaction are as follows:
* E2 is a single step elimination, with a single transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
.
* It is typically undergone by primary substituted alkyl halides, but is possible with some secondary alkyl halides and other compounds.
* The reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
is second order, because it's influenced by both the alkyl halide and the base (bimolecular).
* Because the E2 mechanism results in the formation of a pi bond, the two leaving groups (often a hydrogen and a halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
) need to be antiperiplanar. An antiperiplanar transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
has staggered conformation with lower energy than a synperiplanar transition state which is in eclipsed conformation with higher energy. The reaction mechanism involving staggered conformation is more favorable for E2 reactions (unlike E1 reactions).
* E2 typically uses a strong base. It must be strong enough to remove a weakly acidic hydrogen.
* In order for the pi bond to be created, the hybridization of carbons needs to be lowered from ''sp3'' to ''sp2''.
* The C-H bond is weakened in the rate determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
and therefore a primary deuterium isotope effect much larger than 1 (commonly 2-6) is observed.
* E2 competes with the SN2 reaction mechanism if the base can also act as a nucleophile (true for many common bases).
isobutyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, giv ...
bromide with potassium ethoxide in ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
. The reaction products are isobutene
Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula . It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
Productio ...
, ethanol and potassium bromide
Potassium bromide ( K Br) is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the US. Its action is due to the bromide ion ( sodium bromide is equa ...
.
E1 mechanism
E1 is a model to explain a particular type of chemical elimination reaction. E1 stands for unimolecular elimination and has the following specifications * It is a two-step process of elimination: ''ionization and deprotonation''. **Ionization
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive Electric charge, charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged at ...
: the carbon-halogen bond breaks to give a carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
intermediate.
** deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
of the carbocation.
* E1 typically takes place with tertiary
Tertiary (from Latin, meaning 'third' or 'of the third degree/order..') may refer to:
* Tertiary period, an obsolete geologic period spanning from 66 to 2.6 million years ago
* Tertiary (chemistry), a term describing bonding patterns in organic ch ...
alkyl halides, but is possible with some secondary alkyl halides.
* The reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
is influenced only by the concentration of the alkyl halide because carbocation formation is the slowest step, as known as the rate-determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
. Therefore, first-order kinetics apply (unimolecular).
* The reaction usually occurs in the complete absence of a base or the presence of only a weak base (acidic conditions and high temperature).
* E1 reactions are in competition with SN1 reactions because they share a common carbocationic intermediate.
* A secondary deuterium isotope effect of slightly larger than 1 (commonly 1 - 1.5) is observed.
* There is no antiperiplanar requirement. An example is the pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
of a certain sulfonate ester of menthol
Menthol is an organic compound, specifically a Monoterpene, monoterpenoid, that occurs naturally in the oils of several plants in the Mentha, mint family, such as Mentha arvensis, corn mint and peppermint. It is a white or clear waxy crystallin ...
:
rearrangement reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another at ...
s
An example in ''scheme 2'' is the reaction of tert-butylbromide with potassium ethoxide in ethanol.
E1 eliminations happen with highly substituted alkyl halides for two main reasons.
* Highly substituted alkyl halides are bulky, limiting the room for the E2 one-step mechanism; therefore, the two-step E1 mechanism is favored.
* Highly substituted carbocations are more stable than methyl or primary substituted cations. Such stability gives time for the two-step E1 mechanism to occur.
If SN1 and E1 pathways are competing, the E1 pathway can be favored by increasing the heat.
Specific features :
# Rearrangement possible
# Independent of concentration and basicity of base
Competition among mechanisms
Thereaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
is influenced by the reactivity of halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s, iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency ...
and bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retard ...
being favored. Fluoride is not a good leaving group, so eliminations with fluoride as the leaving group have slower rates than other halogens .
There is a certain level of competition between the elimination reaction and nucleophilic substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile) ...
. More precisely, there are competitions between E2 and SN2 and also between E1 and SN1. Generally, elimination is favored over substitution when
* steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivi ...
around the α-carbon increases.
* a stronger base is used.
* temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
increases (increase entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
)
* the base is a poor nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. Bases with steric bulk, (such as in potassium tert-butoxide
Potassium ''tert''-butoxide (or potassium ''t''-butoxide) is a chemical compound with the formula CH3)3COKsub>''n'' (abbr. KOtBu). This colourless solid is a strong base (pKa of conjugate acid is 17 in H2O), which is useful in organic syn ...
), are often poor nucleophiles.
For example, when a 3° haloalkane is reacts with an alkoxide, due to strong basic character of the alkoxide and unreactivity of 3° group towards SN2, only alkene formation by E2 elimination is observed. Thus, elimination by E2 limits the scope of the Williamson ether synthesis
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ...
(an SN2 reaction) to essentially only 1° haloalkanes; 2° haloalkanes generally do not give synthetically useful yields, while 3° haloalkanes fail completely.
With strong base, 3° haloalkanes give elimination by E2. With weak bases, mixtures of elimination and substitution products form by competing SN1 and E1 pathways.
The case of 2° haloalkanes is relatively complex. For strongly basic nucleophiles (p''K''aH > 11, e.g., hydroxide, alkoxide, acetylide), the result is generally elimination by E2, while weaker bases that are still good nucleophiles (e.g., acetate, azide, cyanide, iodide) will give primarily SN2. Finally, weakly nucleophilic species (e.g., water, alcohols, carboxylic acids) will give a mixture of SN1 and E1.
For 1° haloalkanes with β-branching, E2 elimination is still generally preferred over SN2 for strongly basic nucleophiles. Unhindered 1° haloalkanes favor SN2 when the nucleophile is also unhindered. However, strongly basic and hindered nucleophiles favor E2.
In general, with the exception of reactions in which E2 is impossible because β hydrogens are unavailable (e.g. methyl, allyl, and benzyl halides), clean SN2 substitution is hard to achieve when strong bases are used, as alkene products arising from elimination are almost always observed to some degree. On the other hand, clean E2 can be achieved by simply selecting a sterically hindered base (e.g., potassium ''tert''-butoxide). Similarly, attempts to effect substitution by SN1 almost always result in a product mixture contaminated by some E1 product (again, with the exception of cases where the lack of β hydrogens makes elimination impossible).
In one study the kinetic isotope effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for t ...
(KIE) was determined for the gas phase reaction of several alkyl halides with the chlorate
Chlorate is the common name of the anion, whose chlorine atom is in the +5 oxidation state. The term can also refer to chemical compounds containing this anion, with Chlorate#Compounds (salts), chlorates being the salt (chemistry), salts of chlo ...
ion. In accordance with an E2 elimination the reaction with t-butyl chloride results in a KIE of 2.3. The methyl chloride
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in indus ...
reaction (only SN2 possible) on the other hand has a KIE of 0.85 consistent with a SN2 reaction because in this reaction type the C-H bonds tighten in the transition state. The KIE's for the ethyl (0.99) and isopropyl (1.72) analogues suggest competition between the two reaction modes.
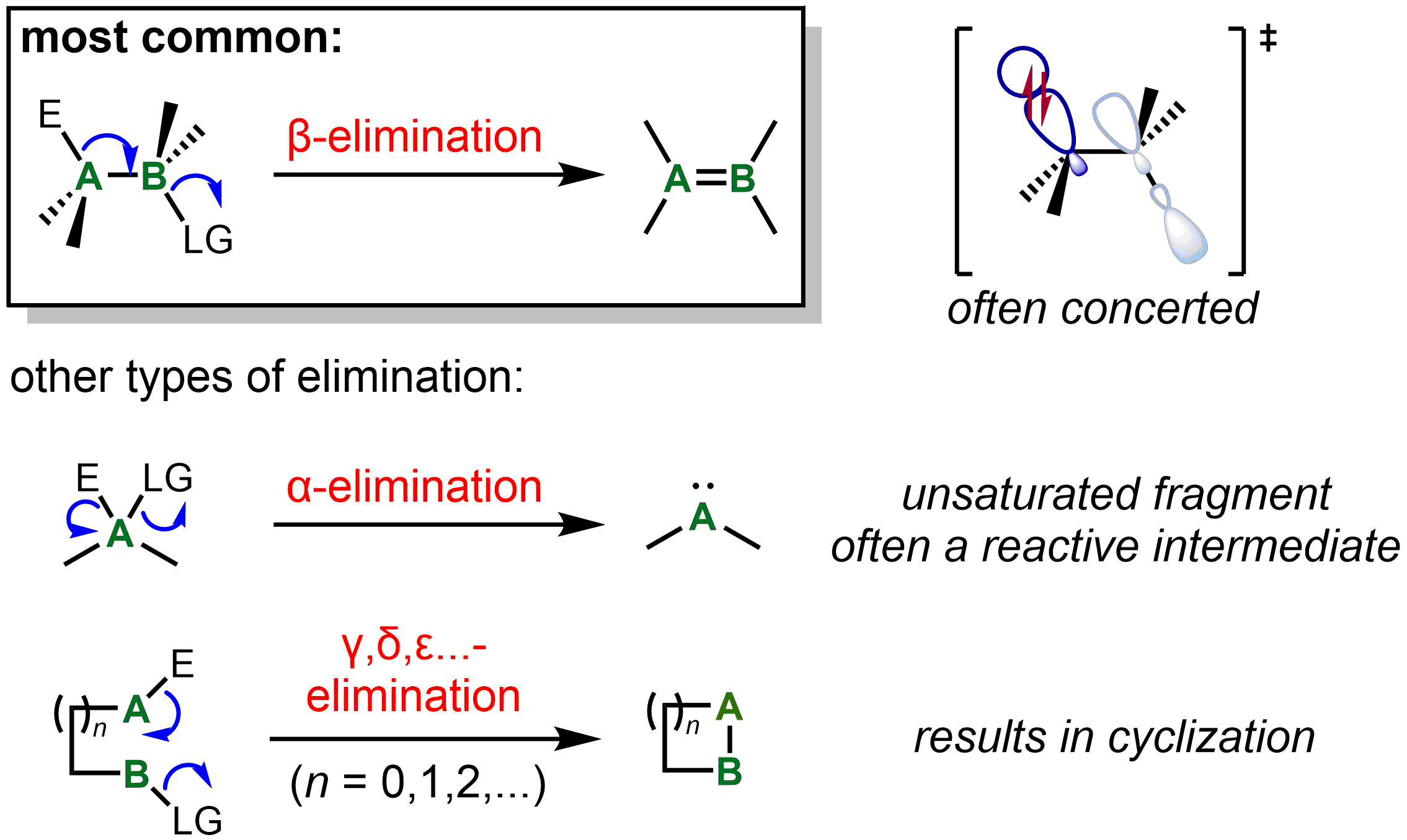
Elimination reactions other than β-elimination
β-Elimination, with loss of electrofuge and nucleofuge on vicinal carbon atoms, is by far the most common type of elimination. The ability to form a stable product containing a C=C or C=X bond, as well as orbital alignment considerations, strongly favors β-elimination over other elimination processes. However, other types are known, generally for systems where β-elimination cannot occur. The next most common type of elimination reaction is α-elimination. For a carbon center, the result of α-elimination is the formation of a carbene, which includes "stable carbenes" such as
The next most common type of elimination reaction is α-elimination. For a carbon center, the result of α-elimination is the formation of a carbene, which includes "stable carbenes" such as carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
or isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragme ...
s. For instance, α-elimination the elements of HCl from chloroform (CHCl3) in the presence of strong base is a classic approach for the generation of dichlorocarbene
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapi ...
, :CCl2, as a reactive intermediate. On the other hand, formic acid undergoes α-elimination to afford the stable products water and carbon monoxide under acidic conditions. α-Elimination may also occur on a metal center, one particularly common result of which is lowering of both the metal oxidation state and coordination number by 2 units in a process known as reductive elimination. (Confusingly, in organometallic terminology, the terms ''α-elimination'' and ''α-abstraction'' refer to processes that result in formation of a metal-carbene complex. In these reactions, it is the carbon adjacent to the metal that undergoes α-elimination.)
In certain special cases, γ- and higher eliminations to form three-membered or larger rings is also possible in both organic and organometallic processes. For instance, certain Pt(II) complexes undergo γ- and δ-elimination to give metallocycles. More recently, γ-silyl elimination of a silylcyclobutyl tosylate has been used to prepare strained bicyclic systems.
History
Many of the concepts and terminology related to elimination reactions were proposed byChristopher Kelk Ingold
Sir Christopher Kelk Ingold (28 October 1893 – 8 December 1970) was a British chemist based in Leeds and London. His groundbreaking work in the 1920s and 1930s on reaction mechanisms and the electronic structure of organic compounds was resp ...
in the 1920s.
See also
* E1cB-elimination reactionReferences
External links
* * {{DEFAULTSORT:Elimination Reaction Olefination reactions Reaction mechanisms