Electron Capture Detector on:
[Wikipedia]
[Google]
[Amazon]
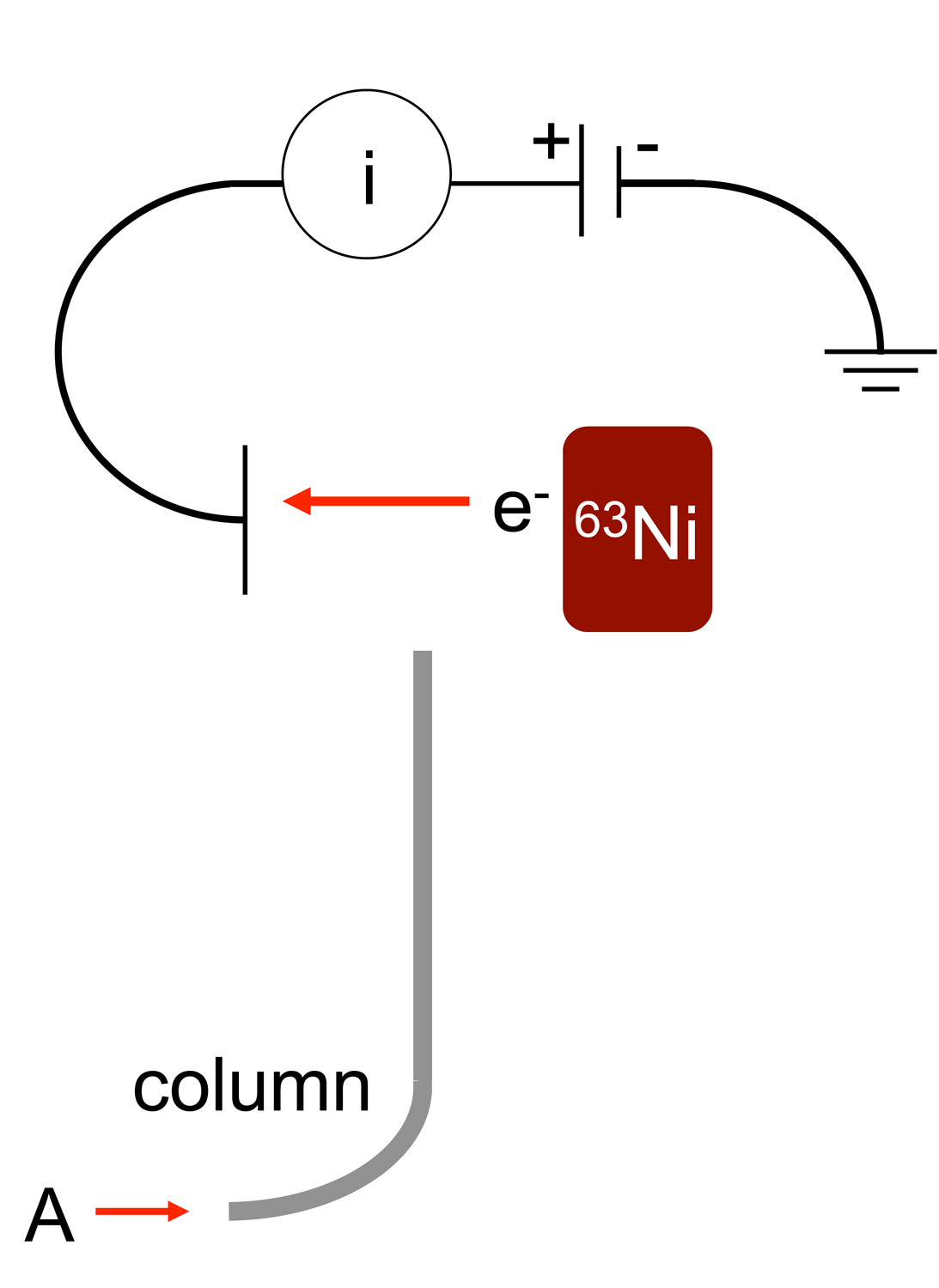 An electron capture detector (ECD) is a device for detecting
An electron capture detector (ECD) is a device for detecting
 An electron capture detector (ECD) is a device for detecting
An electron capture detector (ECD) is a device for detecting atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
and molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
in a gas through the attachment of electrons
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
via electron capture ionization Electron capture ionization is the ionization of a gas phase atom or molecule by attachment of an electron to create an ion of the form A^-. The reaction is
:A + e^- -> ^-
where the M over the arrow denotes that to conserve energy and momentum a t ...
. The device was invented in 1957 by James Lovelock and is used in gas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for Separation process, separating and analyzing compounds that can be vaporized without Chemical decomposition, decomposition. Typical uses of GC include t ...
to detect trace amounts of chemical compounds in a sample.
Gas chromatograph detector
The electron capture detector is used for detecting electron-absorbing components (highelectronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
) such as halogenated compounds in the output stream of a gas chromatograph. The ECD uses a radioactive beta particle
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus, known as beta decay. There are two forms of beta decay, β− decay and � ...
(electron) emitter in conjunction with a so-called makeup gas flowing through the detector chamber. The electron emitter typically consists of a metal foil holding 10 millicuries (370 M Bq) of the radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
. Usually, nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
is used as makeup gas, because it exhibits a low excitation energy, so it is easy to remove an electron from a nitrogen molecule. The electrons emitted from the electron emitter collide with the molecules of the makeup gas, resulting in many more free electrons. The electrons are accelerated towards a positively charged anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
, generating a current. There is therefore always a background signal present in the chromatogram
In chemical analysis, chromatography is a laboratory technique for the Separation process, separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it ...
. As the sample is carried into the detector by the carrier gas, electron-absorbing analyte molecules capture electrons and thereby reduce the current between the collector anode and a cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
. Over a wide range of concentrations the rate of electron capture is proportional to the analyte concentration. ECD detectors are particularly sensitive to halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s, organometallic compounds, nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The name of the compound is composed of a base, which includes the carbon of the , suffixed with "nitrile", so for example is called " propionitrile" (or pr ...
s, or nitro compound
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nit ...
s.
Response mechanism
It is not immediately obvious why the capture of electrons by electronegative analytes reduces the current that flows between the anode and cathode: the molecular negative ions of the analyte carry the same charge as the electrons that were captured. The key to understanding why the current decreases is to ask where charged entities can go ''besides'' being collected at the anode and cathode. The answer is recombination of negative ions or electrons with the positive ions of the makeup gas before these charged entities can be collected at anode and cathode respectively. Negative and positive ions recombine much more rapidly than electrons and positive ions; it is this more rapid neutralization that is the origin of the observed decrease in current. Examination of the rate balance equation with all charge production and loss mechanisms considered reveals that the current collected when the electron capture detector is saturated with analyte is not zero: it is half the current collected when no analyte is present. To laboratory chromatographers this theoretical result is a well known experimental observation.Sensitivity
Depending on the analyte, an ECD can be 10-1000 times more sensitive than a flame ionization detector (FID), and one million times more sensitive than a thermal conductivity detector (TCD). An ECD has a limiteddynamic range
Dynamics (from Greek δυναμικός ''dynamikos'' "powerful", from δύναμις ''dynamis'' " power") or dynamic may refer to:
Physics and engineering
* Dynamics (mechanics), the study of forces and their effect on motion
Brands and ent ...
and finds its greatest application in analysis of halogenated compounds. The detection limit for electron capture detectors is 5 femtograms per second (fg/s) and the detector commonly exhibits a 10,000-fold linear range. This made it possible to detect halogenated compounds such as pesticides and CFCs, even at levels of only one part per trillion ( ppt), thus revolutionizing our understanding of the atmosphere and pollutants.
References
{{Authority control Electron Capture Detector (ECD) Gas chromatography