Chiral Lewis acid on:
[Wikipedia]
[Google]
[Amazon]
Chiral Lewis acids (CLAs) are a type of Lewis acid
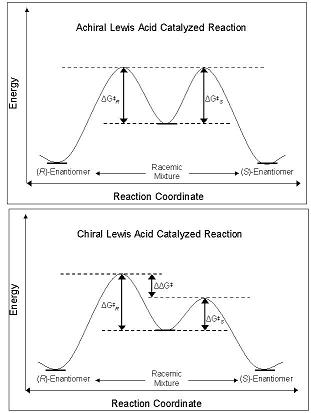 The enantioselectivity of CLAs derives from their ability to perturb the free energy barrier along with the
The enantioselectivity of CLAs derives from their ability to perturb the free energy barrier along with the
 Usually, CLAs are employed to activate the dienophile. A typical CLA catalyst is derived from a Mg2+ center made chiral by attachment of a binol- phosphate ester. CLAs have been applied to a number of intramolecular Diels-Alder reactions.
A complex derived from
Usually, CLAs are employed to activate the dienophile. A typical CLA catalyst is derived from a Mg2+ center made chiral by attachment of a binol- phosphate ester. CLAs have been applied to a number of intramolecular Diels-Alder reactions.
A complex derived from 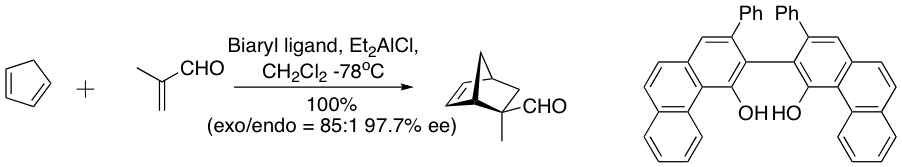 The chiral (acyloxy) borane (CAB) complex is effective in catalyzing a number of aldehyde Diels-Alder reactions. NMR spectroscopic experiments have indicated close proximity of the aldehyde and the aryl ring. Pi stacking between the aryl group and aldehyde has been suggested as an organizational feature that imparts high enantioselectivity to the cycloaddition.
The chiral (acyloxy) borane (CAB) complex is effective in catalyzing a number of aldehyde Diels-Alder reactions. NMR spectroscopic experiments have indicated close proximity of the aldehyde and the aryl ring. Pi stacking between the aryl group and aldehyde has been suggested as an organizational feature that imparts high enantioselectivity to the cycloaddition.
 Bronsted acid-assisted chiral Lewis acid (BLA) catalyzes a number of diene-aldehyde cycloaddition reactions.
Bronsted acid-assisted chiral Lewis acid (BLA) catalyzes a number of diene-aldehyde cycloaddition reactions.
 The transition structures for reactions with both the R and S catalyst enantiomers are:
The transition structures for reactions with both the R and S catalyst enantiomers are:

 The Baylis-Hillman reaction is a route for C-C bond formation between an alpha, beta-unsaturated
The Baylis-Hillman reaction is a route for C-C bond formation between an alpha, beta-unsaturated  The product obtained by the reaction using the chiral
The product obtained by the reaction using the chiral 
 Good enantioselectivity has been observed when a chiral Lewis acid catalyst is used.
Good enantioselectivity has been observed when a chiral Lewis acid catalyst is used.
 The enantioselectivity is believed to be due to the steric interactions between the methyl and
The enantioselectivity is believed to be due to the steric interactions between the methyl and
catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. These acids affect the chirality of the substrate as they react with it. In such reactions, synthesis favors the formation of a specific enantiomer or diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
. The method is an enantioselective asymmetric synthesis reaction. Since they affect chirality, they produce optically active products from optically inactive or mixed starting materials. This type of preferential formation of one enantiomer or diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
over the other is formally known as asymmetric induction. In this kind of Lewis acid, the electron-accepting atom is typically a metal, such as indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts ...
, zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
, lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
, aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
, titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
, or boron. The chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
-altering ligands employed for synthesizing these acids often have multiple Lewis basic sites (often a diol or a dinitrogen structure) that allow the formation of a ring structure involving the metal atom.
Achiral Lewis acids have been used for decades to promote the synthesis of racemic mixtures in myriad different reactions. Since the 1960s, chemists have used Chiral Lewis acids to induce enantioselective reactions. This is useful when the desired product is a specific enantiomer, as is common in drug synthesis. Common reaction types include Diels-Alder reactions, the ene reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile ...
, +2cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". T ...
reactions, hydrocyanation
In organic chemistry, hydrocyanation is a process for conversion of alkenes to nitriles. The reaction involves the addition of hydrogen cyanide and requires a catalyst. This conversion is conducted on an industrial scale for the production of ...
of aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
, and most notably, Sharpless epoxidation
The Sharpless epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols. The oxidizing agent is ''tert''-butyl hydroperoxide. The method relies on a catalyst formed fro ...
s.
Theory
 The enantioselectivity of CLAs derives from their ability to perturb the free energy barrier along with the
The enantioselectivity of CLAs derives from their ability to perturb the free energy barrier along with the reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities. In molecu ...
pathway that leads to either the ''R''- or ''S''- enantiomer. Ground state diastereomers and enantiomers are of equal energy in the ground state, and when reacted with an achiral Lewis acid, their diastereomeric intermediates, transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
s, and products are also of equal energy. This leads to the production of racemic mixtures. However, when a CLA is used in the same reaction, the energetic barrier of formation of one diastereomer is less than that of another; the reaction is under kinetic control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or ...
. If the difference in the energy barriers between the diastereomeric transition states are of sufficient magnitude, then a high enantiomeric excess
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a si ...
of one isomer is observed.
Asymmetric synthesis
Diels-Alder reaction
Diels-Alder reactions occur between a conjugateddiene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. ...
and an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
(commonly known as the dienophile). This cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". T ...
process allows for the stereoselective formation of cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light a ...
rings capable of possessing as many as four contiguous stereogenic centers.
Diels-Alder reactions can lead to the formation of a variety of structural isomers and stereoisomers. Molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecul ...
considers that the endo transition state, instead of the exo transition state, is favored (endo addition rule). Also, augmented secondary orbital interactions have been postulated as the source of enhanced endo diastereoselection.
 Usually, CLAs are employed to activate the dienophile. A typical CLA catalyst is derived from a Mg2+ center made chiral by attachment of a binol- phosphate ester. CLAs have been applied to a number of intramolecular Diels-Alder reactions.
A complex derived from
Usually, CLAs are employed to activate the dienophile. A typical CLA catalyst is derived from a Mg2+ center made chiral by attachment of a binol- phosphate ester. CLAs have been applied to a number of intramolecular Diels-Alder reactions.
A complex derived from diethylaluminium chloride
Diethylaluminium chloride, abbreviated DEAC, is an organoaluminium compound. Although usually given the chemical formula (C2H5)2AlCl, it exists as a dimer, C2H5)2AlClsub>2 It is a precursor to Ziegler-Natta catalysts employed for the production ...
and a “vaulted” biaryl ligand below catalyzes the enantioselective Diels-Alder reaction between cyclopentadiene and methacrolein. The chiral ligand is recovered quantitatively by silica gel chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system ( ...
.
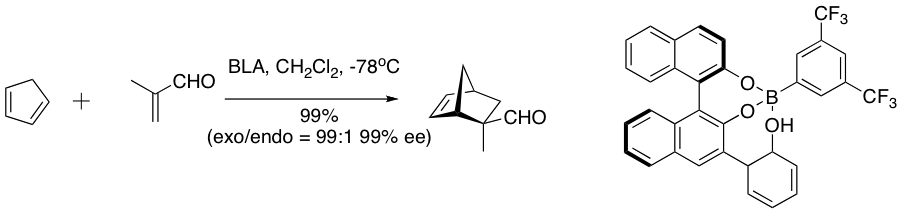
 The chiral (acyloxy) borane (CAB) complex is effective in catalyzing a number of aldehyde Diels-Alder reactions. NMR spectroscopic experiments have indicated close proximity of the aldehyde and the aryl ring. Pi stacking between the aryl group and aldehyde has been suggested as an organizational feature that imparts high enantioselectivity to the cycloaddition.
The chiral (acyloxy) borane (CAB) complex is effective in catalyzing a number of aldehyde Diels-Alder reactions. NMR spectroscopic experiments have indicated close proximity of the aldehyde and the aryl ring. Pi stacking between the aryl group and aldehyde has been suggested as an organizational feature that imparts high enantioselectivity to the cycloaddition.
 Bronsted acid-assisted chiral Lewis acid (BLA) catalyzes a number of diene-aldehyde cycloaddition reactions.
Bronsted acid-assisted chiral Lewis acid (BLA) catalyzes a number of diene-aldehyde cycloaddition reactions.

Aldol reaction
In thealdol
In organic chemistry, an aldol describes a structural motif consisting of a 3-hydroxy ketone or 3-hydroxyaldehyde. Aldols are usually the product of aldol addition. When used alone, the term "aldol" may refer to 3-hydroxybutanal.
Stereochemistry
...
reaction, the diastereoselectivity of the product is often dictated by the geometry of the enolate. The Zimmerman-Traxler model predicts that the Z enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
will give syn products, and that E enolates will give anti products. Reactions catalyzed by tin-based CLAs allow products to deviate from this pattern.
 The transition structures for reactions with both the R and S catalyst enantiomers are:
The transition structures for reactions with both the R and S catalyst enantiomers are:

Baylis-Hillman Reaction
 The Baylis-Hillman reaction is a route for C-C bond formation between an alpha, beta-unsaturated
The Baylis-Hillman reaction is a route for C-C bond formation between an alpha, beta-unsaturated carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
and an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
, which requires a nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
catalyst, usually a tertiary amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
, for a Michael-type addition and elimination. The stereoselectivity of these reactions is usually poor. Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lant ...
(III)-containing CLAs have been demonstrated to improve stereoselectivity. Similarly, a chiral amine may also be used to achieve stereoselectivity.
 The product obtained by the reaction using the chiral
The product obtained by the reaction using the chiral catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
was obtained in good yield with excellent enantioselectivity.

Ene reaction
Chiral Lewis acids have proven useful in theene reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile ...
. When catalyzed by an achiral Lewis acid, the reaction normally provides good diastereoselectivity.
 Good enantioselectivity has been observed when a chiral Lewis acid catalyst is used.
Good enantioselectivity has been observed when a chiral Lewis acid catalyst is used.
 The enantioselectivity is believed to be due to the steric interactions between the methyl and
The enantioselectivity is believed to be due to the steric interactions between the methyl and phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
group, which makes the transition structure of the iso product considerably more favorable.
Achiral Lewis acids in stereoselective synthesis
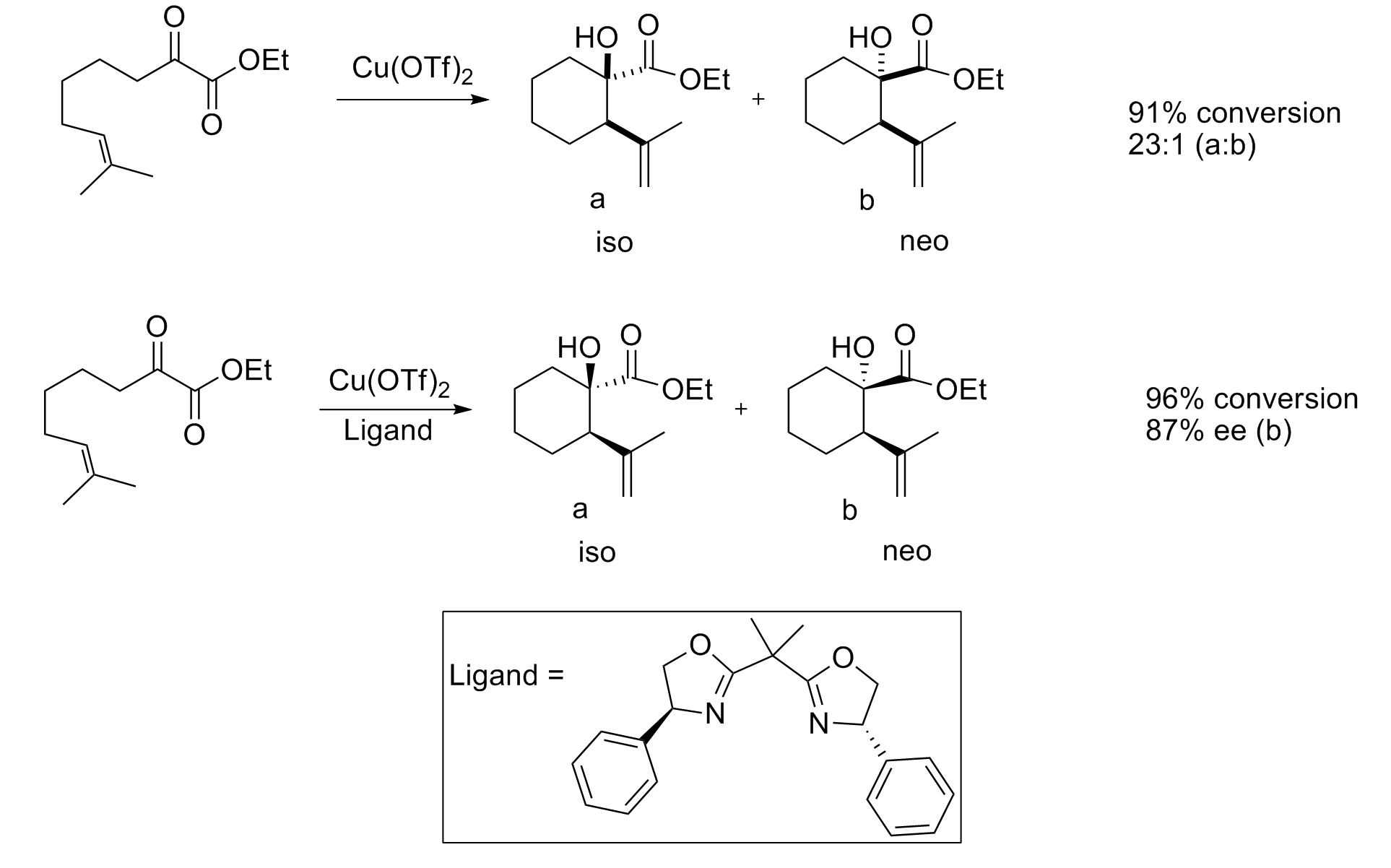
In some cases, an achiral Lewis acid may provide good stereoselectivity. Kimura et al. demonstrated the regio- and diastereoselective coupling of 1,3-diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. ...
s with aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
using a nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
catalyst.
References
{{reflist Stereochemistry Acids