Chemical Formula on:
[Wikipedia]
[Google]
[Amazon]
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or
 Molecular formulae indicate the simple numbers of each type of atom in a molecule of a molecular substance. They are the same as empirical formulae for molecules that only have one atom of a particular type, but otherwise may have larger numbers. An example of the difference is the empirical formula for glucose, which is (''ratio'' 1:2:1), while its molecular formula is (''number of atoms'' 6:12:6). For water, both formulae are . A molecular formula provides more information about a molecule than its empirical formula, but is more difficult to establish.
A molecular formula shows the number of elements in a molecule, and determines whether it is a
Molecular formulae indicate the simple numbers of each type of atom in a molecule of a molecular substance. They are the same as empirical formulae for molecules that only have one atom of a particular type, but otherwise may have larger numbers. An example of the difference is the empirical formula for glucose, which is (''ratio'' 1:2:1), while its molecular formula is (''number of atoms'' 6:12:6). For water, both formulae are . A molecular formula provides more information about a molecule than its empirical formula, but is more difficult to establish.
A molecular formula shows the number of elements in a molecule, and determines whether it is a
 The @ symbol (
The @ symbol (
Hill notation example
from the University of Massachusetts Lowell libraries, including how to sort into Hill system order
Molecular formula calculation applying Hill notation
The library calculating Hill notation i
available on npm
{{DEFAULTSORT:Chemical Formula Chemical nomenclature Notation
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
, using chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structure
A chemical structure determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of ...
s, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., w ...
s, and are generally more limited in power than chemical names and structural formulae.
The simplest types of chemical formulae are called ''empirical formula
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the ...
e'', which use letters and numbers indicating the numerical ''proportions'' of atoms of each type. Molecular formulae indicate the simple numbers of each type of atom in a molecule, with no information on structure. For example, the empirical formula for glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
is (twice as many hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atoms as carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
), while its molecular formula is (12 hydrogen atoms, six carbon and oxygen atoms).
Sometimes a chemical formula is complicated by being written as a condensed formula (or condensed molecular formula, occasionally called a "semi-structural formula"), which conveys additional information about the particular ways in which the atoms are chemically bonded together, either in covalent bonds, ionic bond
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds ...
s, or various combinations of these types. This is possible if the relevant bonding is easy to show in one dimension. An example is the condensed molecular/chemical formula for ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
, which is or . However, even a condensed chemical formula is necessarily limited in its ability to show complex bonding relationships between atoms, especially atoms that have bonds to four or more different substituents.
Since a chemical formula must be expressed as a single line of chemical element symbol
Chemical symbols are the abbreviations used in chemistry for chemical elements, functional groups and chemical compounds. Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with th ...
s, it often cannot be as informative as a true structural formula, which is a graphical representation of the spatial relationship between atoms in chemical compounds (see for example the figure for butane structural and chemical formulae, at right). For reasons of structural complexity, a single condensed chemical formula (or semi-structural formula) may correspond to different molecules, known as isomers. For example, glucose shares its molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
with a number of other sugars, including fructose, galactose
Galactose (, '' galacto-'' + ''-ose'', "milk sugar"), sometimes abbreviated Gal, is a monosaccharide sugar that is about as sweet as glucose, and about 65% as sweet as sucrose. It is an aldohexose and a C-4 epimer of glucose. A galactose molecu ...
and mannose. Linear equivalent chemical ''names'' exist that can and do specify uniquely any complex structural formula (see chemical nomenclature), but such names must use many terms (words), rather than the simple element symbols, numbers, and simple typographical symbols that define a chemical formula.
Chemical formulae may be used in chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
s to describe chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
s and other chemical transformations, such as the dissolving of ionic compounds into solution. While, as noted, chemical formulae do not have the full power of structural formulae to show chemical relationships between atoms, they are sufficient to keep track of numbers of atoms and numbers of electrical charges in chemical reactions, thus balancing chemical equations so that these equations can be used in chemical problems involving conservation of atoms, and conservation of electric charge.
Overview
A chemical formula identifies each constituent element by itschemical symbol
Chemical symbols are the abbreviations used in chemistry for chemical elements, functional groups and chemical compounds. Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with ...
and indicates the proportionate number of atoms of each element. In empirical formulae, these proportions begin with a key element and then assign numbers of atoms of the other elements in the compound, by ratios to the key element. For molecular compounds, these ratio numbers can all be expressed as whole numbers. For example, the empirical formula of ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
may be written because the molecules of ethanol all contain two carbon atoms, six hydrogen atoms, and one oxygen atom. Some types of ionic compounds, however, cannot be written with entirely whole-number empirical formulae. An example is boron carbide, whose formula of is a variable non-whole number ratio with n ranging from over 4 to more than 6.5.
When the chemical compound of the formula consists of simple molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
s, chemical formulae often employ ways to suggest the structure of the molecule. These types of formulae are variously known as ''molecular formulae'' and '' condensed formulae''. A molecular formula enumerates the number of atoms to reflect those in the molecule, so that the molecular formula for glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
is rather than the glucose empirical formula, which is . However, except for very simple substances, molecular chemical formulae lack needed structural information, and are ambiguous.
For simple molecules, a condensed (or semi-structural) formula is a type of chemical formula that may fully imply a correct structural formula. For example, ethanol may be represented by the condensed chemical formula , and dimethyl ether by the condensed formula . These two molecules have the same empirical and molecular formulae (), but may be differentiated by the condensed formulae shown, which are sufficient to represent the full structure of these simple organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
s.
Condensed chemical formulae may also be used to represent ionic compounds that do not exist as discrete molecules, but nonetheless do contain covalently bound clusters within them. These polyatomic ions are groups of atoms that are covalently bound together and have an overall ionic charge, such as the sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
ion. Each polyatomic ion in a compound is written individually in order to illustrate the separate groupings. For example, the compound dichlorine hexoxide has an empirical formula , and molecular formula , but in liquid or solid forms, this compound is more correctly shown by an ionic condensed formula , which illustrates that this compound consists of ions and ions. In such cases, the condensed formula only need be complex enough to show at least one of each ionic species.
Chemical formulae as described here are distinct from the far more complex chemical systematic names that are used in various systems of chemical nomenclature. For example, one systematic name for glucose is (2''R'',3''S'',4''R'',5''R'')-2,3,4,5,6-pentahydroxyhexanal. This name, interpreted by the rules behind it, fully specifies glucose's structural formula, but the name is not a chemical formula as usually understood, and uses terms and words not used in chemical formulae. Such names, unlike basic formulae, may be able to represent full structural formulae without graphs.
Types
Empirical formula
In chemistry, theempirical formula
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the ...
of a chemical is a simple expression of the relative number of each type of atom or ratio of the elements in the compound. Empirical formulae are the standard for ionic compounds, such as , and for macromolecules, such as . An empirical formula makes no reference to isomerism, structure, or absolute number of atoms. The term ''empirical'' refers to the process of elemental analysis, a technique of analytical chemistry
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separati ...
used to determine the relative percent composition of a pure chemical substance by element.
For example, hexane has a molecular formula of , and (for one of its isomers, n-hexane) a structural formula , implying that it has a chain structure of 6 carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
atoms, and 14 hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atoms. However, the empirical formula for hexane is . Likewise the empirical formula for hydrogen peroxide, , is simply , expressing the 1:1 ratio of component elements. Formaldehyde and acetic acid have the same empirical formula, . This is the actual chemical formula for formaldehyde, but acetic acid has double the number of atoms.
Molecular formula
binary compound
In materials chemistry, a binary phase or binary compound is a chemical compound containing two different elements. Some binary phase compounds are molecular, e.g. carbon tetrachloride (CCl4). More typically binary phase refers to extended soli ...
, ternary compound
In inorganic chemistry and materials chemistry, a ternary compound or ternary phase is a chemical compound containing three different elements.
While some ternary compounds are molecular, ''e.g.'' chloroform (), more typically ternary phases r ...
, quaternary compound
In chemistry, a quaternary compound is a compound consisting of exactly four chemical elements.
In another use of the term in organic chemistry, a quaternary compound is or has a cation consisting of a central positively charged atom with four ...
, or has even more elements.
Structural formula
In addition to quantitative description of a molecule, a structural formula captures how the atoms are organized, and shows (or implies) thechemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
s between the atoms. There are multiple types of structural formulas focused on different aspects of the molecular structure.
The two diagrams show two molecules which are structural isomers of each other, since they both have the same molecular formula , but they have different structural formulas as shown.
Condensed formula
Theconnectivity
Connectivity may refer to:
Computing and technology
* Connectivity (media), the ability of the social media to accumulate economic capital from the users connections and activities
* Internet connectivity, the means by which individual terminal ...
of a molecule often has a strong influence on its physical and chemical properties and behavior. Two molecules composed of the same numbers of the same types of atoms (i.e. a pair of isomers) might have completely different chemical and/or physical properties if the atoms are connected differently or in different positions. In such cases, a structural formula is useful, as it illustrates which atoms are bonded to which other ones. From the connectivity, it is often possible to deduce the approximate shape of the molecule.
A condensed (or semi-structural) formula may represent the types and spatial arrangement of bonds in a simple chemical substance, though it does not necessarily specify isomers or complex structures. For example, ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petroc ...
consists of two carbon atoms single-bonded to each other, with each carbon atom having three hydrogen atoms bonded to it. Its chemical formula can be rendered as . In ethylene there is a double bond between the carbon atoms (and thus each carbon only has two hydrogens), therefore the chemical formula may be written: , and the fact that there is a double bond between the carbons is implicit because carbon has a valence of four. However, a more explicit method is to write or less commonly . The two lines (or two pairs of dots) indicate that a double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
connects the atoms on either side of them.
A triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
may be expressed with three lines () or three pairs of dots (), and if there may be ambiguity, a single line or pair of dots may be used to indicate a single bond.
Molecules with multiple functional groups that are the same may be expressed by enclosing the repeated group in round brackets. For example, isobutane may be written . This condensed structural formula implies a different connectivity from other molecules that can be formed using the same atoms in the same proportions ( isomers). The formula implies a central carbon atom connected to one hydrogen atom and three methyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in ma ...
s (). The same number of atoms of each element (10 hydrogens and 4 carbons, or ) may be used to make a straight chain molecule, ''n''- butane: .
Law of composition
In any given chemical compound, the elements always combine in the same proportion with each other. This is thelaw of constant composition
In chemistry, the law of definite proportions, sometimes called Proust's law, or law of constant composition states that a given
chemical compound always contains its component elements in fixed ratio (by mass) and does not depend on its source an ...
.
The law of constant composition says that, in any particular chemical compound, all samples of that compound will be made up of the same elements in the same proportion or ratio. For example, any water molecule is always made up of two hydrogen atoms and one oxygen atom in a 2:1 ratio. If we look at the relative masses of oxygen and hydrogen in a water molecule, we see that 94% of the mass of a water molecule is accounted for by oxygen and the remaining 6% is the mass of hydrogen. This mass proportion will be the same for any water molecule.
Chemical names in answer to limitations of chemical formulae
The alkene called but-2-ene has two isomers, which the chemical formula does not identify. The relative position of the two methyl groups must be indicated by additional notation denoting whether the methyl groups are on the same side of the double bond (''cis'' or ''Z'') or on the opposite sides from each other (''trans'' or ''E''). As noted above, in order to represent the full structural formulae of many complex organic and inorganic compounds, chemical nomenclature may be needed which goes well beyond the available resources used above in simple condensed formulae. SeeIUPAC nomenclature of organic chemistry
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the '' Nomenclature of O ...
and IUPAC nomenclature of inorganic chemistry 2005
Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005 is the 2005 version of '' Nomenclature of Inorganic Chemistry'' (which is informally called the Red Book). It is a collection of rules for naming inorganic compounds, as recommended by ...
for examples. In addition, linear naming systems such as International Chemical Identifier
The International Chemical Identifier (InChI or ) is a textual identifier for chemical substances, designed to provide a standard way to encode molecular information and to facilitate the search for such information in databases and on the we ...
(InChI) allow a computer to construct a structural formula, and simplified molecular-input line-entry system
The simplified molecular-input line-entry system (SMILES) is a specification in the form of a line notation for describing the structure of chemical species using short ASCII strings. SMILES strings can be imported by most molecule editors f ...
(SMILES) allows a more human-readable ASCII input. However, all these nomenclature systems go beyond the standards of chemical formulae, and technically are chemical naming systems, not formula systems.
Polymers in condensed formulae
Forpolymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s in condensed chemical formulae, parentheses are placed around the repeating unit. For example, a hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
molecule that is described as , is a molecule with fifty repeating units. If the number of repeating units is unknown or variable, the letter ''n'' may be used to indicate this formula: .
Ions in condensed formulae
For ions, the charge on a particular atom may be denoted with a right-hand superscript. For example, , or . The total charge on a charged molecule or a polyatomic ion may also be shown in this way, such as for hydronium, , orsulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
, . Note that + and - are used in place of +1 and -1, respectively.
For more complex ions, brackets are often used to enclose the ionic formula, as in , which is found in compounds such as caesium dodecaborate, . Parentheses ( ) can be nested inside brackets to indicate a repeating unit, as in Hexamminecobalt(III) chloride
Hexaamminecobalt(III) chloride is the chemical compound with the formula o(NH3)6l3. It is the chloride salt of the coordination complex o(NH3)6sup>3+, which is considered an archetypal "Werner complex", named after the pioneer of coordination c ...
, . Here, indicates that the ion contains six ammine groups () bonded to cobalt, and encloses the entire formula of the ion with charge +3.
This is strictly optional; a chemical formula is valid with or without ionization information, and Hexamminecobalt(III) chloride may be written as or . Brackets, like parentheses, behave in chemistry as they do in mathematics, grouping terms together they are not specifically employed only for ionization states. In the latter case here, the parentheses indicate 6 groups all of the same shape, bonded to another group of size 1 (the cobalt atom), and then the entire bundle, as a group, is bonded to 3 chlorine atoms. In the former case, it is clearer that the bond connecting the chlorines is ionic, rather than covalent.
Isotopes
Althoughisotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
s are more relevant to nuclear chemistry or stable isotope
The term stable isotope has a meaning similar to stable nuclide, but is preferably used when speaking of nuclides of a specific element. Hence, the plural form stable isotopes usually refers to isotopes of the same element. The relative abundanc ...
chemistry than to conventional chemistry, different isotopes may be indicated with a prefixed superscript in a chemical formula. For example, the phosphate ion containing radioactive phosphorus-32 is . Also a study involving stable isotope ratios might include the molecule .
A left-hand subscript is sometimes used redundantly to indicate the atomic number. For example, for dioxygen, and for the most abundant isotopic species of dioxygen. This is convenient when writing equations for nuclear reactions, in order to show the balance of charge more clearly.
Trapped atoms
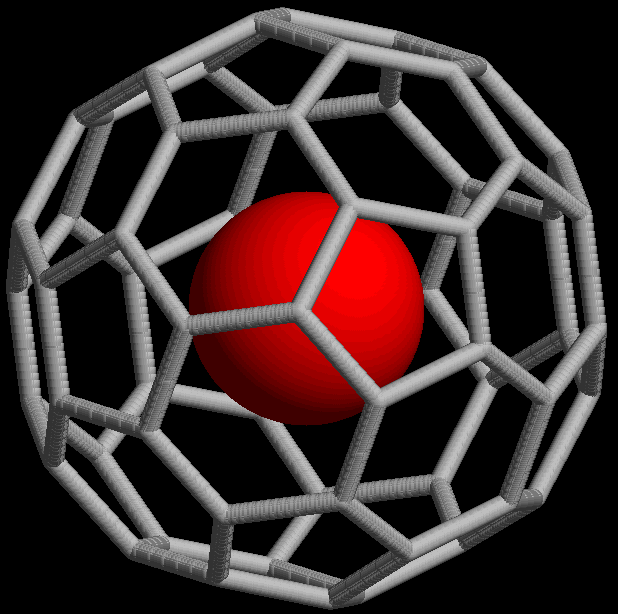
 The @ symbol (
The @ symbol (at sign
The at sign, , is normally read aloud as "at"; it is also commonly called the at symbol, commercial at, or address sign. It is used as an accounting and invoice abbreviation meaning "at a rate of" (e.g. 7 widgets @ £2 per widget = £14), but ...
) indicates an atom or molecule trapped inside a cage but not chemically bound to it. For example, a buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a soccer ball. Each of its 60 carbon atoms is bonded ...
() with an atom (M) would simply be represented as regardless of whether M was inside the fullerene without chemical bonding or outside, bound to one of the carbon atoms. Using the @ symbol, this would be denoted if M was inside the carbon network. A non-fullerene example is , an ion in which one arsenic (As) atom is trapped in a cage formed by the other 32 atoms.
This notation was proposed in 1991 with the discovery of fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
cages ( endohedral fullerenes), which can trap atoms such as La to form, for example, or . The choice of the symbol has been explained by the authors as being concise, readily printed and transmitted electronically (the at sign is included in ASCII
ASCII ( ), abbreviated from American Standard Code for Information Interchange, is a character encoding standard for electronic communication. ASCII codes represent text in computers, telecommunications equipment, and other devices. Because ...
, which most modern character encoding schemes are based on), and the visual aspects suggesting the structure of an endohedral fullerene.
Non-stoichiometric chemical formulae
Chemical formulae most often useinteger
An integer is the number zero (), a positive natural number (, , , etc.) or a negative integer with a minus sign ( −1, −2, −3, etc.). The negative numbers are the additive inverses of the corresponding positive numbers. In the languag ...
s for each element. However, there is a class of compounds, called non-stoichiometric compound
In chemistry, non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); m ...
s, that cannot be represented by small integers. Such a formula might be written using decimal fraction
The decimal numeral system (also called the base-ten positional numeral system and denary or decanary) is the standard system for denoting integer and non-integer numbers. It is the extension to non-integer numbers of the Hindu–Arabic num ...
s, as in , or it might include a variable part represented by a letter, as in , where ''x'' is normally much less than 1.
General forms for organic compounds
A chemical formula used for a series of compounds that differ from each other by a constant unit is called a ''general formula''. It generates a homologous series of chemical formulae. For example, alcohols may be represented by the formula (''n'' ≥ 1), giving the homologs methanol,ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
, propanol
There are two isomers of propanol.
*1-Propanol, ''n''-propanol, or propan-1-ol : CH3CH2CH2OH, the most common meaning
*2-Propanol, Isopropyl alcohol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a c ...
for 1 ≤ ''n'' ≤ 3.
Hill system
The Hill system (or Hill notation) is a system of writing empirical chemical formulae, molecular chemical formulae and components of a condensed formula such that the number ofcarbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
atoms in a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
is indicated first, the number of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atoms next, and then the number of all other chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
s subsequently, in alphabetical order
Alphabetical order is a system whereby character strings are placed in order based on the position of the characters in the conventional ordering of an alphabet. It is one of the methods of collation. In mathematics, a lexicographical order is t ...
of the chemical symbols
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wit ...
. When the formula contains no carbon, all the elements, including hydrogen, are listed alphabetically.
By sorting formulae according to the number of atoms of each element present in the formula according to these rules, with differences in earlier elements or numbers being treated as more significant than differences in any later element or number—like sorting text strings into lexicographical order—it is possible to collate chemical formulae into what is known as Hill system order.
The Hill system was first published by Edwin A. Hill of the United States Patent and Trademark Office in 1900. It is the most commonly used system in chemical databases and printed indexes to sort lists of compounds.Wiggins, Gary. (1991). ''Chemical Information Sources.'' New York: McGraw Hill. p. 120.
A list of formulae in Hill system order is arranged alphabetically, as above, with single-letter elements coming before two-letter symbols when the symbols begin with the same letter (so "B" comes before "Be", which comes before "Br").
The following example formulae are written using the Hill system, and listed in Hill order:
* BrI
* BrClH2Si
* CCl4
* CH3I
* C2H5Br
* H2O4S
See also
* Dictionary of chemical formulae *Formula unit
In chemistry, a formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound. E ...
* Nuclear notation
* Periodic table
* Skeletal formula
* Simplified molecular-input line-entry system
The simplified molecular-input line-entry system (SMILES) is a specification in the form of a line notation for describing the structure of chemical species using short ASCII strings. SMILES strings can be imported by most molecule editors f ...
Notes
References
*External links
*Hill notation example
from the University of Massachusetts Lowell libraries, including how to sort into Hill system order
Molecular formula calculation applying Hill notation
The library calculating Hill notation i
available on npm
{{DEFAULTSORT:Chemical Formula Chemical nomenclature Notation