Carnot efficiency on:
[Wikipedia]
[Google]
[Amazon]
A Carnot cycle is an ideal
 In this case, since it is a reversible thermodynamic cycle (no net change in the system and its surroundings per cycle)
or,
This is true as and are both smaller in magnitude and in fact are in the same ratio as .
In this case, since it is a reversible thermodynamic cycle (no net change in the system and its surroundings per cycle)
or,
This is true as and are both smaller in magnitude and in fact are in the same ratio as .
 The behavior of a Carnot engine or refrigerator is best understood by using a
The behavior of a Carnot engine or refrigerator is best understood by using a 
 Evaluation of the above integral is particularly simple for a Carnot cycle. The amount of energy transferred as work is
The total amount of heat transferred from the hot reservoir to the system (in the isothermal expansion) will be
and the total amount of heat transferred from the system to the cold reservoir (in the isothermal compression) will be
Due to energy conservation, the net heat transferred, , is equal to the work performed
The efficiency is defined to be:
where
* is the work done by the system (energy exiting the system as work),
* < 0 is the heat taken from the system (heat energy leaving the system),
* > 0 is the heat put into the system (heat energy entering the system),
* is the
Evaluation of the above integral is particularly simple for a Carnot cycle. The amount of energy transferred as work is
The total amount of heat transferred from the hot reservoir to the system (in the isothermal expansion) will be
and the total amount of heat transferred from the system to the cold reservoir (in the isothermal compression) will be
Due to energy conservation, the net heat transferred, , is equal to the work performed
The efficiency is defined to be:
where
* is the work done by the system (energy exiting the system as work),
* < 0 is the heat taken from the system (heat energy leaving the system),
* > 0 is the heat put into the system (heat energy entering the system),
* is the
 Carnot's theorem is a formal statement of this fact: ''No engine operating between two heat reservoirs can be more efficient than a Carnot engine operating between those same reservoirs.'' Thus, Equation gives the maximum efficiency possible for any engine using the corresponding temperatures. A corollary to Carnot's theorem states that: ''All reversible engines operating between the same heat reservoirs are equally efficient.'' Rearranging the right side of the equation gives what may be a more easily understood form of the equation, namely that the theoretical maximum efficiency of a heat engine equals the difference in temperature between the hot and cold reservoir divided by the absolute temperature of the hot reservoir. Looking at this formula an interesting fact becomes apparent: Lowering the temperature of the cold reservoir will have more effect on the ceiling efficiency of a heat engine than raising the temperature of the hot reservoir by the same amount. In the real world, this may be difficult to achieve since the cold reservoir is often an existing ambient temperature.
In other words, the maximum efficiency is achieved if and only if entropy does not change per cycle. An entropy change per cycle is made, for example, if there is
Carnot's theorem is a formal statement of this fact: ''No engine operating between two heat reservoirs can be more efficient than a Carnot engine operating between those same reservoirs.'' Thus, Equation gives the maximum efficiency possible for any engine using the corresponding temperatures. A corollary to Carnot's theorem states that: ''All reversible engines operating between the same heat reservoirs are equally efficient.'' Rearranging the right side of the equation gives what may be a more easily understood form of the equation, namely that the theoretical maximum efficiency of a heat engine equals the difference in temperature between the hot and cold reservoir divided by the absolute temperature of the hot reservoir. Looking at this formula an interesting fact becomes apparent: Lowering the temperature of the cold reservoir will have more effect on the ceiling efficiency of a heat engine than raising the temperature of the hot reservoir by the same amount. In the real world, this may be difficult to achieve since the cold reservoir is often an existing ambient temperature.
In other words, the maximum efficiency is achieved if and only if entropy does not change per cycle. An entropy change per cycle is made, for example, if there is
The Steam-Engine and Other Engines
edition 3, page 62, via
Full article (24 page
, also a
Hyperphysics
article on the Carnot cycle. * S. M. Blinde
Carnot Cycle on Ideal Gas
powered by
thermodynamic cycle
A thermodynamic cycle consists of a linked sequence of thermodynamic processes that involve transfer of heat and work into and out of the system, while varying pressure, temperature, and other state variables within the system, and that eventu ...
proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency
Efficiency is the often measurable ability to avoid wasting materials, energy, efforts, money, and time in doing something or in producing a desired result. In a more general sense, it is the ability to do things well, successfully, and without ...
of any classical thermodynamic engine during the conversion of heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is ...
into work
Work may refer to:
* Work (human activity), intentional activity people perform to support themselves, others, or the community
** Manual labour, physical work done by humans
** House work, housework, or homemaking
** Working animal, an animal t ...
, or conversely, the efficiency of a refrigeration
The term refrigeration refers to the process of removing heat from an enclosed space or substance for the purpose of lowering the temperature.International Dictionary of Refrigeration, http://dictionary.iifiir.org/search.phpASHRAE Terminology, ht ...
system in creating a temperature difference through the application of work to the system.
In a Carnot cycle, a system
A system is a group of interacting or interrelated elements that act according to a set of rules to form a unified whole. A system, surrounded and influenced by its environment, is described by its boundaries, structure and purpose and express ...
or engine transfers energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of ...
in the form of heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is ...
between two thermal reservoir
A thermal reservoir, also thermal energy reservoir or thermal bath, is a thermodynamic system with a heat capacity so large that the temperature of the reservoir changes relatively little when a much more significant amount of heat is added or ex ...
s at temperatures and (referred to as the hot and cold reservoirs, respectively), and a part of this transferred energy is converted to the work done by the system. The cycle is reversible, and there is no generation of entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodyna ...
. (In other words, entropy is conserved; entropy is only transferred between the thermal reservoirs and the system without gain or loss of it.) When work is applied to the system, heat moves from the cold to hot reservoir (heat pump
A heat pump is a device that can heat a building (or part of a building) by transferring thermal energy from the outside using a refrigeration cycle. Many heat pumps can also operate in the opposite direction, cooling the building by removing ...
or refrigeration
The term refrigeration refers to the process of removing heat from an enclosed space or substance for the purpose of lowering the temperature.International Dictionary of Refrigeration, http://dictionary.iifiir.org/search.phpASHRAE Terminology, ht ...
). When heat moves from the hot to the cold reservoir, the system applies work to the environment. The work done by the system or engine to the environment per Carnot cycle depends on the temperatures of the thermal reservoirs and the entropy transferred from the hot reservoir to the system per cycle such as , where is heat transferred from the hot reservoir to the system per cycle.
Stages
A Carnot cycle as an idealized thermodynamic cycle performed by a heat engine (Carnot heat engine) consists of the following steps.The pressure–volume graph
When a Carnot cycle is plotted on a pressure–volume diagram (), the isothermal stages follow the isotherm lines for the working fluid, the adiabatic stages move between isotherms, and the area bounded by the complete cycle path represents the total work that can be done during one cycle. From point 1 to 2 and point 3 to 4 the temperature is constant (isothermal process). Heat transfer from point 4 to 1 and point 2 to 3 are equal to zero (adiabatic process).Properties and significance
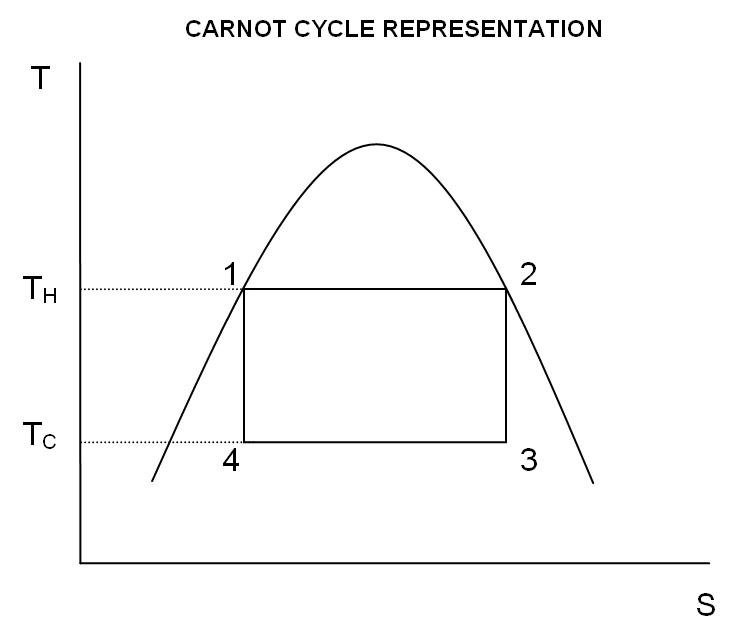
The temperature–entropy diagram
temperature–entropy diagram
In thermodynamics, a temperature–entropy (''T–s'') diagram is a thermodynamic diagram used to visualize changes to temperature () and specific entropy () during a thermodynamic process or cycle as the graph of a curve. It is a useful and ...
(''T''–''S'' diagram), in which the thermodynamic state is specified by a point on a graph with entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodyna ...
(''S'') as the horizontal axis and temperature (''T'') as the vertical axis (). For a simple closed system (control mass analysis), any point on the graph represents a particular state of the system. A thermodynamic process is represented by a curve connecting an initial state (A) and a final state (B). The area under the curve is:
which is the amount heat transferred in the process. If the process moves the system to greater entropy, the area under the curve is the amount of heat absorbed by the system in that process; otherwise, it is the amount of heat removed from or leaving from the system. For any cyclic process, there is an upper portion of the cycle and a lower portion. In ''T''-''S'' diagrams for a clockwise cycle, the area under the upper portion will be the energy absorbed by the system during the cycle, while the area under the lower portion will be the energy removed from the system during the cycle. The area inside the cycle is then the difference between the two (the absorbed net heat energy), but since the internal energy of the system must have returned to its initial value, this difference must be the amount of work done by the system per cycle. Referring to , mathematically, for a reversible process, we may write the amount of work done over a cyclic process as:
Since ''dU'' is an exact differential
In multivariate calculus, a differential or differential form is said to be exact or perfect (''exact differential''), as contrasted with an inexact differential, if it is equal to the general differential dQ for some differentiable function ...
, its integral over any closed loop is zero and it follows that the area inside the loop on a ''T''–''S'' diagram is equal to the total work performed by the system on the surroundings if the loop is traversed in a clockwise direction, and is equal to the total work done on the system by the surroundings as the loop is traversed in a counterclockwise direction.

The Carnot cycle
absolute temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.
Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic ...
of the cold reservoir, and
* is the absolute temperature of the hot reservoir.
* is the maximum system entropy
* is the minimum system entropy
The expression with the temperature can be derived from the expressions above with the entropy: and . Since , a minus sign appears in the final expression for .
This is the Carnot heat engine working efficiency definition as the fraction of the work done by the system to the thermal energy received by the system from the hot reservoir per cycle. This thermal energy is the cycle initiator.
Reversed Carnot cycle
A Carnot heat-engine cycle described is a totally reversible cycle. That is, all the processes that compose it can be reversed, in which case it becomes the Carnot heat pump and refrigeration cycle. This time, the cycle remains exactly the same except that the directions of any heat and work interactions are reversed. Heat is absorbed from the low-temperature reservoir, heat is rejected to a high-temperature reservoir, and a work input is required to accomplish all this. The ''P''–''V'' diagram of the reversed Carnot cycle is the same as for the Carnot heat-engine cycle except that the directions of the processes are reversed.Carnot's theorem
It can be seen from the above diagram that for any cycle operating between temperatures and , none can exceed the efficiency of a Carnot cycle.friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and material elements sliding against each other. There are several types of friction:
*Dry friction is a force that opposes the relative lateral motion of ...
leading to dissipation
In thermodynamics, dissipation is the result of an irreversible process that takes place in homogeneous thermodynamic systems. In a dissipative process, energy ( internal, bulk flow kinetic, or system potential) transforms from an initial form to ...
of work into heat. In that case, the cycle is not reversible and the Clausius theorem becomes an inequality rather than an equality. Otherwise, since entropy is a state function
In the thermodynamics of equilibrium, a state function, function of state, or point function for a thermodynamic system is a mathematical function relating several state variables or state quantities (that describe equilibrium states of a system ...
, the required dumping of heat into the environment to dispose of excess entropy leads to a (minimal) reduction in efficiency. So Equation gives the efficiency of any reversible heat engine
In thermodynamics and engineering, a heat engine is a system that converts heat to mechanical energy, which can then be used to do mechanical work. It does this by bringing a working substance from a higher state temperature to a lower stat ...
.
In mesoscopic heat engines, work per cycle of operation in general fluctuates due to thermal noise. If the cycle is performed quasi-statically, the fluctuations vanish even on the mesoscale. However, if the cycle is performed faster than the relaxation time of the working medium, the fluctuations of work are inevitable. Nevertheless, when work and heat fluctuations are counted, an exact equality relates the exponential average of work performed by any heat engine to the heat transfer from the hotter heat bath.
Efficiency of real heat engines
Carnot realized that, in reality, it is not possible to build a thermodynamically reversible engine. So, real heat engines are even less efficient than indicated by Equation . In addition, real engines that operate along the Carnot cycle style (isothermal expansion / isentropic expansion / isothermal compression / isentropic compression) are rare. Nevertheless, Equation is extremely useful for determining the maximum efficiency that could ever be expected for a given set of thermal reservoirs. Although Carnot's cycle is an idealization, Equation as the expression of the Carnot efficiency is still useful. Consider theaverage
In ordinary language, an average is a single number taken as representative of a list of numbers, usually the sum of the numbers divided by how many numbers are in the list (the arithmetic mean). For example, the average of the numbers 2, 3, 4, 7 ...
temperatures,
at which the first integral is over a part of a cycle where heat goes into the system and the second integral is over a cycle part where heat goes out from the system. Then, replace ''TH'' and ''TC'' in Equation by ⟨''TH''⟩ and ⟨''TC''⟩, respectively, to estimate the efficiency a heat engine.
For the Carnot cycle, or its equivalent, the average value ⟨''TH''⟩ will equal the highest temperature available, namely ''TH'', and ⟨''TC''⟩ the lowest, namely ''TC''. For other less efficient thermodynamic cycles, ⟨''TH''⟩ will be lower than ''TH'', and ⟨''TC''⟩ will be higher than ''TC''. This can help illustrate, for example, why a reheater
An afterburner (or reheat in British English) is an additional combustion component used on some jet engines, mostly those on military aircraft, military supersonic aircraft. Its purpose is to increase thrust, usually for supersonic flight, ...
or a regenerator
Regenerator may refer to:
* Regenerative heat exchanger, a type of heat exchanger
* Regenerator (band), an independent record label set up in 2001
* Regenerator (''Resident Evil''), an enemy creature in the 2005 video game ''Resident Evil 4''
* ' ...
can improve the thermal efficiency of steam power plants and why the thermal efficiency of combined-cycle power plants (which incorporate gas turbines operating at even higher temperatures) exceeds that of conventional steam plants. The first prototype of the diesel engine
The diesel engine, named after Rudolf Diesel, is an internal combustion engine in which ignition of the fuel is caused by the elevated temperature of the air in the cylinder due to mechanical compression; thus, the diesel engine is a so-cal ...
was based on the Carnot cycle.
Carnot heat engine as an impractical macroscopic construct
A Carnot heat engine is a heat engine performing a Carnot cycle, and its realization on a macroscopic scale is impractical. For example, for the isothermal expansion part of the Carnot cycle, the following conditions must be satisfied simultaneously at every step in the expansion: * The hot reservoir temperature ''TH'' is infinitesimally higher than the system gas temperature ''T'' so heat flow (energy transfer) from the hot reservoir to the gas is made without increasing ''T'' (via infinitesimal work on the surroundings by the gas as another energy transfer); if ''TH'' is significantly higher than ''T'', then ''T'' may be not uniform through the gas so the system would deviate from thermal equilibrium as well as not being a reversible process (i.e. not a Carnot cycle) or ''T'' might increase noticeably so it would not be an isothermal process. * The force externally applied on the piston (opposite to the internal force on the piston by the gas) needs to be infinitesimally reduced somehow. Without this external assistance, it would not be possible to follow a gas PV (Pressure-Volume) curve downward at a constant ''T'' since following this curve means that the gas-to-piston force decreases (''P'' decreases) as the volume expands (the piston moves outward). If this assistance is so strong that the volume expansion is significant, the system may deviate from thermal equilibrium as well as not being a reversible process (i.e. not a Carnot cycle). These (and other) "infinitesimal" requirements make the Carnot cycle take an infinite amount of time. Other practical requirements that make the Carnot cycle hard to realize (e.g., fine control of the gas, thermal contact with the surroundings including high and low temperature reservoirs), so the Carnot engine should be thought as the theoretical limit of macroscopic scale heat engines rather than a practical device that could ever be built.See also
*Carnot heat engine
A Carnot heat engine is a heat engine that operates on the Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1 ...
* Reversible process (thermodynamics)
In thermodynamics, a reversible process is a process, involving a system and its surroundings, whose direction can be reversed by infinitesimal changes in some properties of the surroundings, such as pressure or temperature.
Throughout an ...
References
;Notes ;Sources :* Carnot, Sadi,Reflections on the Motive Power of Fire
''Reflections on the Motive Power of Fire and on Machines Fitted to Develop that Power'' is a book published in 1824 by French physicist Sadi Carnot.full text of 1897 ed. ( Full text of 1897 edition on Wikisource ) The 118-page book's French ti ...
:* Ewing, J. A. (1910The Steam-Engine and Other Engines
edition 3, page 62, via
Internet Archive
The Internet Archive is an American digital library with the stated mission of "universal access to all knowledge". It provides free public access to collections of digitized materials, including websites, software applications/games, music, ...
:*
:*
:*
:* American Institute of Physics, 2011. . Abstract atFull article (24 page
, also a
External links
Hyperphysics
article on the Carnot cycle. * S. M. Blinde
Carnot Cycle on Ideal Gas
powered by
Wolfram Mathematica
Wolfram Mathematica is a software system with built-in libraries for several areas of technical computing that allow machine learning, statistics, symbolic computation, data manipulation, network analysis, time series analysis, NLP, optimizat ...
{{Thermodynamic cycles, state=uncollapsed
Thermodynamic cycles