Carnot's theorem (thermodynamics) on:
[Wikipedia]
[Google]
[Amazon]
In
 The proof of the Carnot theorem is a
The proof of the Carnot theorem is a
thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws ...
, Carnot's theorem, developed in 1824 by Nicolas Léonard Sadi Carnot
''Sous-lieutenant'' Nicolas Léonard Sadi Carnot (; 1 June 1796 – 24 August 1832) was a French mechanical engineer in the French Army, military scientist and physicist, and often described as the "father of thermodynamics". He published o ...
, also called Carnot's rule, is a principle that specifies limits on the maximum efficiency
Efficiency is the often measurable ability to avoid wasting materials, energy, efforts, money, and time in doing something or in producing a desired result. In a more general sense, it is the ability to do things well, successfully, and without ...
that any heat engine
In thermodynamics and engineering, a heat engine is a system that converts heat to mechanical energy, which can then be used to do mechanical work. It does this by bringing a working substance from a higher state temperature to a lower stat ...
can obtain.
Carnot's theorem states that all heat engines operating between the same two thermal or heat reservoirs can't have efficiencies greater than a reversible heat engine operating between the same reservoirs. A corollary
In mathematics and logic, a corollary ( , ) is a theorem of less importance which can be readily deduced from a previous, more notable statement. A corollary could, for instance, be a proposition which is incidentally proved while proving another ...
of this theorem is that every reversible heat engine operating between a pair of heat reservoirs is equally efficient, regardless of the working substance employed or the operation details. Since a Carnot heat engine
A Carnot heat engine is a heat engine that operates on the Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1 ...
is also a reversible engine, the efficiency of all the reversible heat engines is determined as the efficiency of the Carnot heat engine that depends solely on the temperatures of its hot and cold reservoirs.
The maximum efficiency (i.e., the Carnot heat engine efficiency) of a heat engine operating between cold and hot reservoirs, denoted as and respectively, is the ratio of the temperature difference between the reservoirs to the hot reservoir temperature, expressed in the equation
:
where and are the absolute temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.
Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic ...
s of the hot and cold reservoirs, respectively, and the efficiency is the ratio of the work
Work may refer to:
* Work (human activity), intentional activity people perform to support themselves, others, or the community
** Manual labour, physical work done by humans
** House work, housework, or homemaking
** Working animal, an animal t ...
done by the engine (to the surroundings
Surroundings are the area around a given physical or geographical point or place. The exact definition depends on the field. Surroundings can also be used in geography (when it is more precisely known as vicinity, or vicinage) and mathematics, ...
) to the heat drawn out of the hot reservoir (to the engine).
is greater than zero if and only if there is a temperature difference between the two thermal reservoirs. Since is the upper limit of all reversible and irreversible heat engine efficiencies, it is concluded that work from a heat engine can be produced if and only if there is a temperature difference between two thermal reservoirs connecting to the engine.
Carnot's theorem is a consequence of the second law of thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unle ...
. Historically, it was based on contemporary caloric theory
The caloric theory is an obsolete scientific theory that heat consists of a self-repellent fluid called caloric that flows from hotter bodies to colder bodies. Caloric was also thought of as a weightless gas that could pass in and out of pores ...
, and preceded the establishment of the second law.
Proof
 The proof of the Carnot theorem is a
The proof of the Carnot theorem is a proof by contradiction
In logic and mathematics, proof by contradiction is a form of proof that establishes the truth or the validity of a proposition, by showing that assuming the proposition to be false leads to a contradiction. Proof by contradiction is also known ...
or reductio ad absurdum
In logic, (Latin for "reduction to absurdity"), also known as (Latin for "argument to absurdity") or ''apagogical arguments'', is the form of argument that attempts to establish a claim by showing that the opposite scenario would lead to absu ...
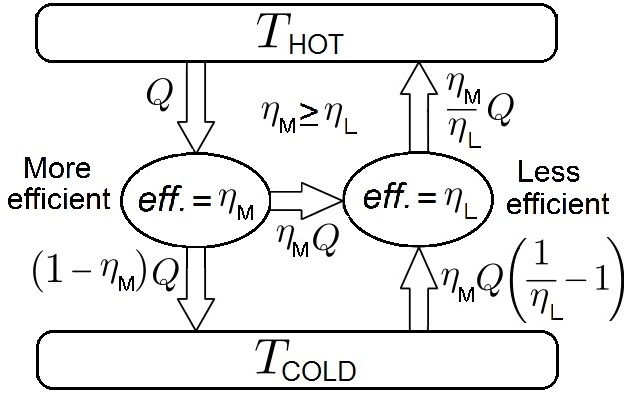
(a method to prove a statement by assuming its falsity and logically deriving a false or contradictory statement from this assumption), based on a situation like the right figure where two heat engines with different efficiencies are operating between two thermal reservoirs at different temperature. The relatively hotter reservoir is called the hot reservoir and the other reservoir is called the cold reservoir. A (not necessarily reversible) heat engine with a greater efficiency is driving a reversible heat engine with a less efficiency , causing the latter to act as a heat pump
A heat pump is a device that can heat a building (or part of a building) by transferring thermal energy from the outside using a refrigeration cycle. Many heat pumps can also operate in the opposite direction, cooling the building by removing ...
. The requirement for the engine to be reversible is necessary to explain work and heat associated with it by using its known efficiency. However, since , the net heat flow would be backwards, i.e., into the hot reservoir:
:
where represents heat, for input to an object denoted by the subscript, for output from an object denoted by the subscript, and for the hot thermal reservoir. If heat flows from the hot reservoir then it has the sign of + while if flows to the hot reservoir then it has the sign of +. This expression can be easily derived by using the definition of the efficiency
Efficiency is the often measurable ability to avoid wasting materials, energy, efforts, money, and time in doing something or in producing a desired result. In a more general sense, it is the ability to do things well, successfully, and without ...
of a heat engine, , where work and heat in this expression are net quantities per engine cycle, and the conservation of energy for each engine as shown below. The sign convention of work , with which the sign of + for work done by an engine to its surroundings, is employed.
The above expression means that heat into the hot reservoir from the engine pair (can be considered as a single engine) is greater than heat into the engine pair from the hot reservoir (i.e., the hot reservoir continuously gets energy). A reversible heat engine with a low efficiency delivers more heat (energy) to the hot reservoir for a given amount of work (energy) to this engine when it is being driven as a heat pump. All these mean that heat can transfer from cold to hot places without external work, and such a heat transfer is impossible by the second law of thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unle ...
.
* It may seem odd that a hypothetical reversible heat pump with a low efficiency is used to violate the second law of thermodynamics, but the figure of merit
A figure of merit is a quantity used to characterize the performance of a device, system or method, relative to its alternatives. Examples
*Clock rate of a CPU
*Calories per serving
*Contrast ratio of an LCD
*Frequency response of a speaker
* Fi ...
for refrigerator units is not the efficiency, , but the coefficient of performance
The coefficient of performance or COP (sometimes CP or CoP) of a heat pump, refrigerator or air conditioning system is a ratio of useful heating or cooling provided to work (energy) required. Higher COPs equate to higher efficiency, lower energy ( ...
(COP), which is where this has the sign opposite to the above (+ for work done to the engine).
Let's find the values of work and heat depicted in the right figure in which a reversible heat engine with a less efficiency is driven as a heat pump by a heat engine with a more efficiency .
The definition of the efficiency
Efficiency is the often measurable ability to avoid wasting materials, energy, efforts, money, and time in doing something or in producing a desired result. In a more general sense, it is the ability to do things well, successfully, and without ...
is for each engine and the following expressions can be made:
:
:
The denominator of the second expression, , is made to make the expression to be consistent, and it helps to fill the values of work and heat for the engine .
For each engine, the absolute value of the energy entering the engine, , must be equal to the absolute value of the energy leaving from the engine, . Otherwise, energy is continuously accumulated in an engine or the conservation of energy is violated by taking more energy from an engine than input energy to the engine:
:
:
In the second expression, is used to find the term describing the amount of heat taken from the cold reservoir, completing the absolute value expressions of work and heat in the right figure.
Having established that the right figure values are correct, Carnot's theorem may be proven for irreversible and the reversible heat engines as shown below.
Reversible engines
To see that every reversible engine operating between reservoirs at temperatures and must have the same efficiency, assume that two reversible heat engines have different efficiencies, and let the relatively more efficient engine drive the relatively less efficient engine as a heat pump. As the right figure shows, this will cause heat to flow from the cold to the hot reservoir without external work, which violates the second law of thermodynamics. Therefore, both (reversible) heat engines have the same efficiency, and we conclude that: :''All reversible heat engines that operate between the same two thermal'' (''heat'') ''reservoirs have the same efficiency.'' The reversible heat engine efficiency can be determined by analyzing a Carnot heat engine as one of reversible heat engine. This conclusion is an important result because it helps establish the Clausius theorem, which implies that the change inentropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodyna ...
is unique for all reversible processes:
:
as the entropy change, that is made during a transition from a thermodynamic equilibrium
Thermodynamic equilibrium is an axiomatic concept of thermodynamics. It is an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In the ...
state to a state in a ''V-T'' (Volume-Temperature) space, is the same over all reversible process paths between these two states. If this integral were not path independent, then entropy would not be a state variable
A state variable is one of the set of variables that are used to describe the mathematical "state" of a dynamical system. Intuitively, the state of a system describes enough about the system to determine its future behaviour in the absence of a ...
.
Irreversible engines
Let's think two engines, one is that is relatively more efficient irreversible engine while the other is that is relatively less efficient reversible engine, and we construct a machine described in the right figure ( drives as a heat pump). Then this machine violates the second law of thermodynamics. Since a Carnot heat engine is a reversible heat engine, with the conclusion in the discussion about two reversible heat engines above, we have the first part of Carnot's theorem: :''No irreversible heat engine is more efficient than a Carnot heat engine operating between the same two thermal reservoirs.''Definition of thermodynamic temperature
The efficiency of a heat engine is the work done by the engine divided by the heat introduced to the engine per engine cycle or where is the work done by the engine, is the heat to the cold reservoir from the engine, and is the heat to the engine from the hot reservoir, per cycle. Thus, the efficiency depends only on . The sign of ''qC'' > 0 for the waste heat lost by the system violates the sign convention ofheat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is ...
.
Because all reversible heat engines operating between temperatures and must have the same efficiency, the efficiency of a reversible heat engine is a function of only the two reservoir temperatures:
In addition, a reversible heat engine operating between temperatures and must have the same efficiency as one consisting of two cycles, one between and another (intermediate) temperature , and the second between and (). This can only be the case if
Specializing to the case that is a fixed reference temperature: the temperature of the triple point of water as 273.16. (Of course any reference temperature and any positive numerical value could be used — the choice here corresponds to the Kelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and ...
scale.) Then for any and ,
:
Therefore, if thermodynamic temperature is defined by
:
then the function viewed as a function of thermodynamic temperature, is
:
It follows immediately that
Substituting this equation back into the above equation gives a relationship for the efficiency in terms of thermodynamic temperatures:
Applicability to fuel cells and batteries
Sincefuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most bat ...
s and batteries
Battery most often refers to:
* Electric battery, a device that provides electrical power
* Battery (crime), a crime involving unlawful physical contact
Battery may also refer to:
Energy source
*Automotive battery, a device to provide power t ...
can generate useful power when all components of the system are at the same temperature (), they are clearly not limited by Carnot's theorem, which states that no power can be generated when . This is because Carnot's theorem applies to engines converting thermal energy to work, whereas fuel cells and batteries instead convert chemical energy to work. Nevertheless, the second law of thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unle ...
still provides restrictions on fuel cell and battery energy conversion.
A Carnot battery is a type of energy storage system that stores electricity in heat storage and converts the stored heat back to electricity through thermodynamic cycles.
See also
* Chambadal–Novikov efficiency * Heating and cooling efficiency boundsReferences
{{DEFAULTSORT:Carnot's Theorem (Thermodynamics) Laws of thermodynamics Physics theorems Thought experiments in physics