Conjugation (biochemistry) on:
[Wikipedia]
[Google]
[Amazon]
 Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a
Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a
 The introduction of nucleophilic catalyst can significantly enhance reaction rate (shown in Figure 7). For example, using
The introduction of nucleophilic catalyst can significantly enhance reaction rate (shown in Figure 7). For example, using  Recent developments that exploit proximal functional groups have enabled hydrazone condensations to operate at 20 M−1s−1 at neutral pH while oxime condensations have been discovered which proceed at 500-10000 M−1s−1 at neutral pH without added catalysts.
Recent developments that exploit proximal functional groups have enabled hydrazone condensations to operate at 20 M−1s−1 at neutral pH while oxime condensations have been discovered which proceed at 500-10000 M−1s−1 at neutral pH without added catalysts.
 Contrasting with the classic Staudinger reaction, Staudinger ligation is a second order reaction in which the rate-limiting step is the formation of phosphazide (specific reaction mechanism shown in Figure 9). The triphenylphosphine first reacts with the azide to yield an azaylide through a four-membered ring
Contrasting with the classic Staudinger reaction, Staudinger ligation is a second order reaction in which the rate-limiting step is the formation of phosphazide (specific reaction mechanism shown in Figure 9). The triphenylphosphine first reacts with the azide to yield an azaylide through a four-membered ring 
 An improved reaction developed by chemist Karl Barry Sharpless involves the copper (I) catalyst, which couples azide with terminal alkyne that only give 1,4 substituted 1,2,3 triazoles in high yields (shown below in Figure 11). The mechanistic study suggests a stepwise reaction. The Cu (I) first couples with acetylenes, and then it reacts with azide to generate a six-membered intermediate. The process is very robust that it occurs at pH ranging from 4 to 12, and copper (II) sulfate is often used as a catalyst in the presence of a
An improved reaction developed by chemist Karl Barry Sharpless involves the copper (I) catalyst, which couples azide with terminal alkyne that only give 1,4 substituted 1,2,3 triazoles in high yields (shown below in Figure 11). The mechanistic study suggests a stepwise reaction. The Cu (I) first couples with acetylenes, and then it reacts with azide to generate a six-membered intermediate. The process is very robust that it occurs at pH ranging from 4 to 12, and copper (II) sulfate is often used as a catalyst in the presence of a 

 * Ir-catalyzed Lys and N-terminus (reductive) alkylation
Imines formed from the condensation of aldehydes with lysines or the N-terminus can be reduced efficient by an water-stable p*Ir(bipy)(H2O)O4 complex in the presence of formate ions (serving as the hydride source). The reaction happens readily under physiologically relevant conditions and results in high conversion for various aromatic aldehydes.
* Pd-catalyzed Tyr O-alkylation
By using a pre-formed electrophilic π-allylpalladium(II) reagent derived from allylic acetate or carbamate precursors, selective allylic alkylation of tyrosines can be achieved in aqueous solution at room temperature and in the presence of cysteines.
* Ir-catalyzed Lys and N-terminus (reductive) alkylation
Imines formed from the condensation of aldehydes with lysines or the N-terminus can be reduced efficient by an water-stable p*Ir(bipy)(H2O)O4 complex in the presence of formate ions (serving as the hydride source). The reaction happens readily under physiologically relevant conditions and results in high conversion for various aromatic aldehydes.
* Pd-catalyzed Tyr O-alkylation
By using a pre-formed electrophilic π-allylpalladium(II) reagent derived from allylic acetate or carbamate precursors, selective allylic alkylation of tyrosines can be achieved in aqueous solution at room temperature and in the presence of cysteines.
 * Au-catalyzed Cys alkylation
Cysteine-containing peptides have been shown to undergo 1,2-addition to allenes in the presence of gold(I) and/or silver(I) salts, producing hydroxyl substituted vinyl thioethers. The reaction with peptides proceeds with high yields and is selective for cysteines over other nucleophilic residues.
However, the reactivity towards proteins is much decreased, potentially due to the coordination of gold to the protein backbone.
* Au-catalyzed Cys alkylation
Cysteine-containing peptides have been shown to undergo 1,2-addition to allenes in the presence of gold(I) and/or silver(I) salts, producing hydroxyl substituted vinyl thioethers. The reaction with peptides proceeds with high yields and is selective for cysteines over other nucleophilic residues.
However, the reactivity towards proteins is much decreased, potentially due to the coordination of gold to the protein backbone.

 * Cys arylation
Free thiols has been considered unfavorable for Pd-mediated reactions due to Pd-catalyst decomposition. However, PdII oxidative addition complexes (OACs) supported by dialkylbiaryl phosphine ligands have shown to work efficiently towards cysteine S-arylation.
The first example is the use of PdII OAC with RuPhos: The PdII complex resulting from the oxidative addition of aryl halides or trifluoromethanesulfonates and using RuPhos as the ligand could chemoselectively modify cysteines in various buffer with 5% organic co-solvent under neutral pH. This method has been shown to modify peptides and proteins, achieve peptide macrocyclization (by using bis-palladium reagent and peptides with two unprotected cysteines) and synthesizing antibody-drug conjugates (ADCs). Changing the ligand to sSPhos supports the PdII complex to be sufficiently water soluble to achieve cysteine S-arylation under cosolvent-free aqueous conditions.
There are other applications of this method where the PdII complexes were generated as PdII-peptide OACs by introducing 4-halophenylalanine into peptides during SPPS to achieve peptide-peptide or peptide-protein ligation.
* Cys arylation
Free thiols has been considered unfavorable for Pd-mediated reactions due to Pd-catalyst decomposition. However, PdII oxidative addition complexes (OACs) supported by dialkylbiaryl phosphine ligands have shown to work efficiently towards cysteine S-arylation.
The first example is the use of PdII OAC with RuPhos: The PdII complex resulting from the oxidative addition of aryl halides or trifluoromethanesulfonates and using RuPhos as the ligand could chemoselectively modify cysteines in various buffer with 5% organic co-solvent under neutral pH. This method has been shown to modify peptides and proteins, achieve peptide macrocyclization (by using bis-palladium reagent and peptides with two unprotected cysteines) and synthesizing antibody-drug conjugates (ADCs). Changing the ligand to sSPhos supports the PdII complex to be sufficiently water soluble to achieve cysteine S-arylation under cosolvent-free aqueous conditions.
There are other applications of this method where the PdII complexes were generated as PdII-peptide OACs by introducing 4-halophenylalanine into peptides during SPPS to achieve peptide-peptide or peptide-protein ligation.
 Alternate to directly oxidative addition to the peptide, the Pd OACs could also be transferred to the protein through amine-selective acylation reaction via NHS ester. The latter has been applied to selectively label surface lysine residues of a protein (forming PdII-protein OACs) and oligonucleotides (forming PdII-oligonucleotide OACs), which could then be linked to cysteine-containing peptides or proteins.
Alternate to directly oxidative addition to the peptide, the Pd OACs could also be transferred to the protein through amine-selective acylation reaction via NHS ester. The latter has been applied to selectively label surface lysine residues of a protein (forming PdII-protein OACs) and oligonucleotides (forming PdII-oligonucleotide OACs), which could then be linked to cysteine-containing peptides or proteins.
 Another example of protein-protein cross-coupling is achieved through converting cysteine residues into an electrophilic S-aryl–Pd–X OAC by utilizing an intramolecular oxidative addition strategy.
* Lys arylation
Similar to cysteine, lysine N-arylation could be achieved through Pd OACs with different dialkylbiaryl phosphine ligands. Due to weaker nucleophilicity and slower reductive elimination rate compared to cysteine, the selection of supporting ligands is shown to be critical. The bulky BrettPhos and ''t''-BuBrettPhos ligands in conjunction with mildly basic sodium phenoxide have been used as the strategy to functionalize lysines on peptide substrates. The reaction happens in mild conditions and is selective over most other nucleophilic amino acid residues.
Another example of protein-protein cross-coupling is achieved through converting cysteine residues into an electrophilic S-aryl–Pd–X OAC by utilizing an intramolecular oxidative addition strategy.
* Lys arylation
Similar to cysteine, lysine N-arylation could be achieved through Pd OACs with different dialkylbiaryl phosphine ligands. Due to weaker nucleophilicity and slower reductive elimination rate compared to cysteine, the selection of supporting ligands is shown to be critical. The bulky BrettPhos and ''t''-BuBrettPhos ligands in conjunction with mildly basic sodium phenoxide have been used as the strategy to functionalize lysines on peptide substrates. The reaction happens in mild conditions and is selective over most other nucleophilic amino acid residues.


biomolecule
A biomolecule or biological molecule is loosely defined as a molecule produced by a living organism and essential to one or more typically biological processes. Biomolecules include large macromolecules such as proteins, carbohydrates, lipids ...
. Methods to conjugate biomolecules are applied in various field, including medicine, diagnostics, biocatalysis and materials. Synthetically modified biomolecules can have diverse functionalities, such as tracking cellular events, revealing enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
function, determining protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
biodistribution, imaging
Imaging is the representation or reproduction of an object's form; especially a visual representation (i.e., the formation of an image).
Imaging technology is the application of materials and methods to create, preserve, or duplicate images.
...
specific biomarkers
In biomedical contexts, a biomarker, or biological marker, is a measurable indicator of some biological state or condition. Biomarkers are often measured and evaluated using blood, urine, or soft tissues to examine normal biological processes, p ...
, and delivering drugs
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via inhalation, injection, smoking, ingestio ...
to targeted cells.
Bioconjugation is a crucial strategy that links these modified biomolecules with different substrates. Besides applications in biomedical research, bioconjugation has recently also gained importance in nanotechnology
Nanotechnology is the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). At this scale, commonly known as the nanoscale, surface area and quantum mechanical effects become important in describing propertie ...
such as bioconjugated quantum dots
Quantum dots (QDs) or semiconductor nanocrystals are semiconductor particles a few nanometres in size with optical and electronic properties that differ from those of larger particles via quantum mechanical effects. They are a central topic i ...
.
The most common types of bioconjugation include coupling of a small molecule (such as biotin
Biotin (also known as vitamin B7 or vitamin H) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. ...
or a fluorescent dye) to a protein. Antibody-drug conjugates such as Brentuximab vedotin and Gemtuzumab ozogamicin are examples falling into this category. Other less common molecules used in bioconjugation are oligosaccharides
An oligosaccharide (; ) is a saccharide polymer containing a small number (typically three to ten) of monosaccharides (simple sugars). Oligosaccharides can have many functions including cell recognition and cell adhesion.
They are normally presen ...
, nucleic acids
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nucleic a ...
, synthetic polymers such as polyethylene glycol
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular wei ...
, and carbon nanotubes
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range (nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
* ''Single-walled carbon nanotubes'' (''SWC ...
. Protein-protein conjugations, such as the coupling of an antibody to an enzyme, or the linkage of protein complexes, is also facilitated via bioconjugations.
Common Bioconjugation Reactions
Synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organi ...
of bioconjugates involves a variety of challenges, ranging from the simple and nonspecific use of a fluorescent dye
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescence, fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromaticity, aromatic groups, or planar o ...
marker to the complex design of antibody drug conjugates. Various bioconjugation reactions have been developed to chemically modify proteins. Common types of bioconjugation reactions on proteins are coupling of lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. Lysine contains an α-amino group (which is in the protonated form when the lysine is dissolved in water at physiological pH), an α-carboxylic acid group ( ...
, cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
, and tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is ...
amino acid residues
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, which are the monomers of the polymer. A single amino acid m ...
, as well as modification of tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromat ...
residues and of the N- and C- terminus.
However, these reactions often lack chemoselectivity Chemoselectivity is the preferential reaction of a chemical reagent with one of two or more different functional groups.
In a chemoselective system, a reagent in the presence of an aldehyde and an ester would mostly target the aldehyde, even if it ...
and efficiency, because they depend on the presence of native amino acids, which are present in large quantities that hinder selectivity. There is an increasing need for chemical strategies that can effectively attach synthetic molecules site specifically to proteins. One strategy is to first install a unique functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
onto a protein, and then a bioorthogonal reaction is used to couple a biomolecule with this unique functional group. The bioorthogonal reactions targeting non-native functional groups are widely used in bioconjugation chemistry. Some important reactions are modification of ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
and aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
, Staudinger ligation with organic azide
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ (anatomy), organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or ...
s, copper-catalyzed Huisgen cycloaddition of azides, and strain promoted Huisgen cycloaddition of azides.
On Natural Amino Acids
Reactions of lysines
The nucleophilic lysine residue is commonly targeted site in protein bioconjugation, typically throughamine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
-reactive ''N''-hydroxysuccinimidyl (NHS) esters
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ...
. To obtain optimal number of deprotonated lysine residues, the pH of the aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water ...
must be below the pKa of the lysine ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
group, which is around 10.5, so the typical pH of the reaction is about 8 and 9. The common reagent for the coupling reaction is NHS-ester (shown in the first reaction below in Figure 1), which reacts with nucleophilic lysine through a lysine acylation
In chemistry, acylation is a broad class of chemical reactions in which an acyl group () is added to a substrate. The compound providing the acyl group is called the acylating agent. The substrate to be acylated and the product include the foll ...
mechanism. Other similar reagents are isocyanates
In organic chemistry, isocyanate is the functional group with the formula . Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyan ...
and isothiocyanates
In organic chemistry, isothiocyanate is a functional group as found in compounds with the formula . Isothiocyanates are the more common isomers of organic thiocyanates, thiocyanates, which have the formula .
Occurrence
Many isothiocyanates fr ...
that undergo a similar mechanism (shown in the second and third reactions in Figure 1 below). Benzoyl fluorides (shown in the last reaction below in Figure 1), which allows for lysine modification of proteins under mild conditions (low temperature, physiological pH), were recently proposed as an alternative to classically used lysine specific reagents.
Reactions of cysteines
Because free cysteine rarely occurs on protein surface, it is an excellent choice for chemoselective modification. Under basic condition, the cysteine residues will be deprotonated to generate athiolate
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
nucleophile, which will react with soft electrophiles, such as maleimides
Maleimide is a chemical compound with the chemical formula, formula H2C2(CO)2NH (see diagram). This unsaturated imide is an important building block in organic synthesis. The name is a contraction of maleic acid and imide, the -C(O)NHC(O)- functi ...
and iodoacetamides (shown in the first two reactions in Figure 2 below). As a result, a carbon-sulfur bond is formed. Another modification of cysteine residues involves the formation of disulfide bond
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inor ...
(shown in the third reaction in Figure 2). The reduced cysteine residues react with exogenous disulfides, generating new a disulfide bond on the protein. An excess of disulfides is often used to drive the reaction, such as 2-thiopyridone and 3-carboxy-4-nitrothiophenol. Electron-deficient alkynes
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
were demonstrated to selectively react with cysteine residues of proteins in the presence of other nucleophilic amino acid residues. Depending on the alkyne substitution, these reactions can produce either cleavable (when alkynone derivatives are used), or hydrolytically stable bioconjugates (when 3-arylpropiolonitriles
3-Arylpropiolonitriles (APN) belong to a class of electron-deficient alkyne derivatives substituted by two electron-withdrawing groups – a nitrile and an aryl moieties. Such activation results in improved selectivity towards highly reactive thio ...
are used; the last reaction below in Figure 2).
Reactions of tyrosines
Tyrosine residues are relatively unreactive; therefore they have not been a popular targets for bioconjugation. Recent development has shown that the tyrosine can be modified throughelectrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitut ...
s (EAS) reactions, and it is selective for the aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
carbon adjacent to the phenolic hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
group. This becomes particularly useful in the case that cysteine residues cannot be targeted. Specifically, diazonium effectively couples with tyrosine residues (diazonium salt
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent, comp ...
shown as reagent in the first reaction in Figure 3 below), and an electron withdrawing substituent in the 4-position of diazonium salt can effectively increase the efficiency of the reaction. Cyclic diazodicarboxyamide derivative like 4-Phenyl-1,2,4-triazole-3,5-dione (PTAD) were reported for selective bioconjugation on tyrosine residues (the second reaction in Figure 3 below). A three-component Mannich-type reaction with aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
and aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...
s (the last reaction in Figure 3) was also described to be relatively tyrosine-selective under mild optimised reaction conditions.
Reactions of N- and C- termini
Since natural amino acid residues are usually present in large quantities, it is often difficult to modify one single site. Strategies targeting the termini of protein have been developed, because they greatly enhanced the site selectivity of protein modification. One of the N- termini modifications involves the functionalization of the terminal amino acid. Theoxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of N-terminal serine
Serine
(symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − ...
and threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− ...
residues are able to generate N-terminal aldehyde, which can undergo further bioorthogonal reactions (shown in the first reaction in Figure 4). Another type of modification involves the condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
of N-terminal cysteine with aldehyde, generating thiazolidine that is stable at high pH (second reaction in Figure 4). Using pyridoxal phosphate
Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependen ...
(PLP), several N-terminal amino acids can undergo transamination
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids.This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential a ...
to yield N-terminal aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
, such as glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (G ...
and aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of protei ...
(third reaction in Figure 4).
An example of C-termini modification is the native chemical ligation Native Chemical Ligation (NCL) is an important extension of the chemical ligation concept for constructing a larger polypeptide chain by the covalent condensation of two or more unprotected peptides segments. Native chemical ligation is the most ...
(NCL), which is the coupling between a C-terminal thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix ...
and a N-terminal cysteine (Figure 5).
Bioorthogonal Reactions: On Unique Functional Groups
Modification of ketones and aldehydes
A ketone or aldehyde can be attached to a protein through the oxidation of N-terminal serine residues or transamination with PLP. Additionally, they can be introduced by incorporating unnatural amino acids via the Tirrell method or Schultz method. They will then selectively condense with an alkoxyamine and ahydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
, producing oxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general Chemical formula, formula , where R is an organic Side chain, side-chain and R' may be hydrogen, forming an aldoxime, or another organic functional g ...
and hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. ...
derivatives (shown in the first and second reactions, respectively, in Figure 6). This reaction is highly chemoselective in terms of protein bioconjugation, but the reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
is slow. The mechanistic studies show that the rate determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
is the dehydration
In physiology, dehydration is a lack of total body water that disrupts metabolic processes. It occurs when free water loss exceeds intake, often resulting from excessive sweating, health conditions, or inadequate consumption of water. Mild deh ...
of tetrahedral intermediate
A tetrahedral intermediate is a reaction intermediate in which the bond arrangement around an initially double-bonded carbon atom has been transformed from trigonal to tetrahedral. Tetrahedral intermediates result from nucleophilic addition to a c ...
, so a mild acidic
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.
The first category of acids are the ...
solution is often employed to accelerate the dehydration step.
 The introduction of nucleophilic catalyst can significantly enhance reaction rate (shown in Figure 7). For example, using
The introduction of nucleophilic catalyst can significantly enhance reaction rate (shown in Figure 7). For example, using aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...
as a nucleophilic catalyst, a less populated protonated carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
becomes a highly populated protonated Schiff base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldim ...
. In other words, it generates a high concentration of reactive electrophile. The oxime ligation can then occur readily, and it has been reported that the rate increased up to 400 times under mild acidic condition. The key of this catalyst is that it can generate a reactive electrophile without competing with desired product.
 Recent developments that exploit proximal functional groups have enabled hydrazone condensations to operate at 20 M−1s−1 at neutral pH while oxime condensations have been discovered which proceed at 500-10000 M−1s−1 at neutral pH without added catalysts.
Recent developments that exploit proximal functional groups have enabled hydrazone condensations to operate at 20 M−1s−1 at neutral pH while oxime condensations have been discovered which proceed at 500-10000 M−1s−1 at neutral pH without added catalysts.
Staudinger ligation with azides
The Staudinger ligation of azides andphosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
has been used extensively in field of chemical biology. Because it is able to form a stable amide bond in living cells and animals, it has been applied to modification of cell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
, ''in vivo'' imaging, and other bioconjugation studies.
 Contrasting with the classic Staudinger reaction, Staudinger ligation is a second order reaction in which the rate-limiting step is the formation of phosphazide (specific reaction mechanism shown in Figure 9). The triphenylphosphine first reacts with the azide to yield an azaylide through a four-membered ring
Contrasting with the classic Staudinger reaction, Staudinger ligation is a second order reaction in which the rate-limiting step is the formation of phosphazide (specific reaction mechanism shown in Figure 9). The triphenylphosphine first reacts with the azide to yield an azaylide through a four-membered ring transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
, and then an intramolecular reaction leads to the iminophosphorane intermediate, which will then give the amide-linkage under hydrolysis.

Huisgen cyclization of azides
= Copper catalyzed Huisgen cyclization of azides
= Azide has become a popular target for chemoselective protein modification, because they are small in size and have a favorable thermodynamic reaction potential. One such azide reactions is the +2cycloaddition reaction withalkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
, but the reaction requires high temperature and often gives mixtures of regioisomers.
 An improved reaction developed by chemist Karl Barry Sharpless involves the copper (I) catalyst, which couples azide with terminal alkyne that only give 1,4 substituted 1,2,3 triazoles in high yields (shown below in Figure 11). The mechanistic study suggests a stepwise reaction. The Cu (I) first couples with acetylenes, and then it reacts with azide to generate a six-membered intermediate. The process is very robust that it occurs at pH ranging from 4 to 12, and copper (II) sulfate is often used as a catalyst in the presence of a
An improved reaction developed by chemist Karl Barry Sharpless involves the copper (I) catalyst, which couples azide with terminal alkyne that only give 1,4 substituted 1,2,3 triazoles in high yields (shown below in Figure 11). The mechanistic study suggests a stepwise reaction. The Cu (I) first couples with acetylenes, and then it reacts with azide to generate a six-membered intermediate. The process is very robust that it occurs at pH ranging from 4 to 12, and copper (II) sulfate is often used as a catalyst in the presence of a reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
.

= Strain promoted Huisgen cyclization of azides
= Even though Staudinger ligation is a suitable bioconjugation in living cells without major toxicity, the phosphine's sensitivity to air oxidation and its poorsolubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
in water significantly hinder its efficiency. The copper(I) catalyzed azide-alkyne coupling has reasonable reaction rate and efficiency under physiological conditions, but copper poses significant toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacteria, bacterium, or plant, as well as the effect o ...
and sometimes interferes with protein functions in living cells. In 2004, chemist Carolyn R. Bertozzi's lab developed a metal free +2cycloaddition using strained cyclooctyne and azide
In chemistry, azide (, ) is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
. Cyclooctyne, which is the smallest stable cycloalkyne, can couple with azide through +2cycloaddition, leading to two regioisomeric triazole
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial Isomer, isomerism, depending on the positioning of the nitrogen atoms w ...
s (Figure 12). The reaction occurs readily at room temperature
Room temperature, colloquially, denotes the range of air temperatures most people find comfortable indoors while dressed in typical clothing. Comfortable temperatures can be extended beyond this range depending on humidity, air circulation, and ...
and therefore can be used to effectively modify living cells without negative effects. It has also been reported that the installation of fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
substituents on a cyclic alkyne can greatly accelerate the reaction rate.

Transition Metal-Mediated Bioconjugation Reactions
Transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
-based bioconjugation had been challenging due to the nature of biological conditions – aqueous solution, room temperature, mild pH, and low substrate concentrations – which are generally challenging for organometallic reactions. However, recently, besides copper-catalyzed + 2azide alkyne cycloaddition reaction, more and more diverse transition metal-mediated chemical transformations have been applied for bioconjugation reactions, introducing olefin metathesis, alkylation, C–H arylation, C–C, C–S, and C–N cross-coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two different fragments are joined. Cross-couplings are a subset of the more general coupling reactions. Often cross-coupling reactions require metal catalysts. One important re ...
s.
Alkylation
On Natural Amino Acids
* Rh-catalyzed Trp and Cys alkylation Using in situ generated RhII-carbenoid by activation of vinyl-substituted diazo compounds with Rh2(OAc)4, tryptophans and cysteines were shown to be selectively alkylated in aqueous media. However, this method is limited to surface tryptophans and cysteines possibly because of steric constraints. * Ir-catalyzed Lys and N-terminus (reductive) alkylation
Imines formed from the condensation of aldehydes with lysines or the N-terminus can be reduced efficient by an water-stable p*Ir(bipy)(H2O)O4 complex in the presence of formate ions (serving as the hydride source). The reaction happens readily under physiologically relevant conditions and results in high conversion for various aromatic aldehydes.
* Pd-catalyzed Tyr O-alkylation
By using a pre-formed electrophilic π-allylpalladium(II) reagent derived from allylic acetate or carbamate precursors, selective allylic alkylation of tyrosines can be achieved in aqueous solution at room temperature and in the presence of cysteines.
* Ir-catalyzed Lys and N-terminus (reductive) alkylation
Imines formed from the condensation of aldehydes with lysines or the N-terminus can be reduced efficient by an water-stable p*Ir(bipy)(H2O)O4 complex in the presence of formate ions (serving as the hydride source). The reaction happens readily under physiologically relevant conditions and results in high conversion for various aromatic aldehydes.
* Pd-catalyzed Tyr O-alkylation
By using a pre-formed electrophilic π-allylpalladium(II) reagent derived from allylic acetate or carbamate precursors, selective allylic alkylation of tyrosines can be achieved in aqueous solution at room temperature and in the presence of cysteines.
 * Au-catalyzed Cys alkylation
Cysteine-containing peptides have been shown to undergo 1,2-addition to allenes in the presence of gold(I) and/or silver(I) salts, producing hydroxyl substituted vinyl thioethers. The reaction with peptides proceeds with high yields and is selective for cysteines over other nucleophilic residues.
However, the reactivity towards proteins is much decreased, potentially due to the coordination of gold to the protein backbone.
* Au-catalyzed Cys alkylation
Cysteine-containing peptides have been shown to undergo 1,2-addition to allenes in the presence of gold(I) and/or silver(I) salts, producing hydroxyl substituted vinyl thioethers. The reaction with peptides proceeds with high yields and is selective for cysteines over other nucleophilic residues.
However, the reactivity towards proteins is much decreased, potentially due to the coordination of gold to the protein backbone.

Arylation
On Natural Amino Acids
* Trp arylation Multiple methods have been reported to achieve tryptophan C–H arylation, where diverse electrophiles such as aryl halides and aryl boronic acids (an example shown below) have been used to transfer the aryl groups. However, current tryptophan C–H arylation reaction conditions remain relatively harsh, requiring organic solvents, low pH and/or high temperatures. * Cys arylation
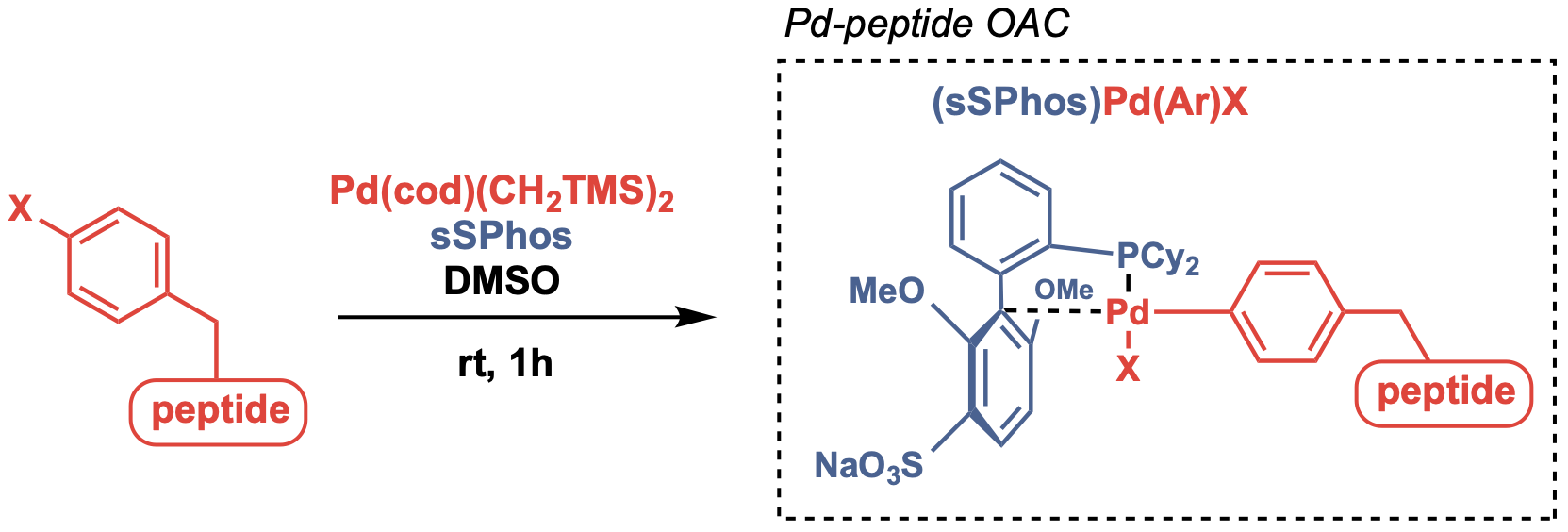
Free thiols has been considered unfavorable for Pd-mediated reactions due to Pd-catalyst decomposition. However, PdII oxidative addition complexes (OACs) supported by dialkylbiaryl phosphine ligands have shown to work efficiently towards cysteine S-arylation.
The first example is the use of PdII OAC with RuPhos: The PdII complex resulting from the oxidative addition of aryl halides or trifluoromethanesulfonates and using RuPhos as the ligand could chemoselectively modify cysteines in various buffer with 5% organic co-solvent under neutral pH. This method has been shown to modify peptides and proteins, achieve peptide macrocyclization (by using bis-palladium reagent and peptides with two unprotected cysteines) and synthesizing antibody-drug conjugates (ADCs). Changing the ligand to sSPhos supports the PdII complex to be sufficiently water soluble to achieve cysteine S-arylation under cosolvent-free aqueous conditions.
There are other applications of this method where the PdII complexes were generated as PdII-peptide OACs by introducing 4-halophenylalanine into peptides during SPPS to achieve peptide-peptide or peptide-protein ligation.
* Cys arylation
Free thiols has been considered unfavorable for Pd-mediated reactions due to Pd-catalyst decomposition. However, PdII oxidative addition complexes (OACs) supported by dialkylbiaryl phosphine ligands have shown to work efficiently towards cysteine S-arylation.
The first example is the use of PdII OAC with RuPhos: The PdII complex resulting from the oxidative addition of aryl halides or trifluoromethanesulfonates and using RuPhos as the ligand could chemoselectively modify cysteines in various buffer with 5% organic co-solvent under neutral pH. This method has been shown to modify peptides and proteins, achieve peptide macrocyclization (by using bis-palladium reagent and peptides with two unprotected cysteines) and synthesizing antibody-drug conjugates (ADCs). Changing the ligand to sSPhos supports the PdII complex to be sufficiently water soluble to achieve cysteine S-arylation under cosolvent-free aqueous conditions.
There are other applications of this method where the PdII complexes were generated as PdII-peptide OACs by introducing 4-halophenylalanine into peptides during SPPS to achieve peptide-peptide or peptide-protein ligation.
 Alternate to directly oxidative addition to the peptide, the Pd OACs could also be transferred to the protein through amine-selective acylation reaction via NHS ester. The latter has been applied to selectively label surface lysine residues of a protein (forming PdII-protein OACs) and oligonucleotides (forming PdII-oligonucleotide OACs), which could then be linked to cysteine-containing peptides or proteins.
Alternate to directly oxidative addition to the peptide, the Pd OACs could also be transferred to the protein through amine-selective acylation reaction via NHS ester. The latter has been applied to selectively label surface lysine residues of a protein (forming PdII-protein OACs) and oligonucleotides (forming PdII-oligonucleotide OACs), which could then be linked to cysteine-containing peptides or proteins.
 Another example of protein-protein cross-coupling is achieved through converting cysteine residues into an electrophilic S-aryl–Pd–X OAC by utilizing an intramolecular oxidative addition strategy.
* Lys arylation
Similar to cysteine, lysine N-arylation could be achieved through Pd OACs with different dialkylbiaryl phosphine ligands. Due to weaker nucleophilicity and slower reductive elimination rate compared to cysteine, the selection of supporting ligands is shown to be critical. The bulky BrettPhos and ''t''-BuBrettPhos ligands in conjunction with mildly basic sodium phenoxide have been used as the strategy to functionalize lysines on peptide substrates. The reaction happens in mild conditions and is selective over most other nucleophilic amino acid residues.
Another example of protein-protein cross-coupling is achieved through converting cysteine residues into an electrophilic S-aryl–Pd–X OAC by utilizing an intramolecular oxidative addition strategy.
* Lys arylation
Similar to cysteine, lysine N-arylation could be achieved through Pd OACs with different dialkylbiaryl phosphine ligands. Due to weaker nucleophilicity and slower reductive elimination rate compared to cysteine, the selection of supporting ligands is shown to be critical. The bulky BrettPhos and ''t''-BuBrettPhos ligands in conjunction with mildly basic sodium phenoxide have been used as the strategy to functionalize lysines on peptide substrates. The reaction happens in mild conditions and is selective over most other nucleophilic amino acid residues.

On Unnatural Amino Acids
Pd-mediated Sonogashira, Heck, and Suzuki-Miyaura cross-coupling reactions have been applied widely to modify peptides and proteins, where diverse Pd reagents have been developed for the application in aqueous solutions. Those reactions require the protein or peptide substrate bearing unnatural functional groups such as alkyne, aryl halides, and aryl boronic acids, which can be achieved through genetic code expansion or post-translational modifications.
Examples of Applied Bioconjugation Techniques
Growth Factors
Bioconjugation of TGF-β to iron oxide nanoparticles and its activation through magnetic hyperthermia in-vitro has been reported. This was done by using 1-(3-dimethylaminopropyl)ethylcarbodiimide combined with N-Hydroxysuccinimide to form primary amide bonds with the free primary amines on the growth factor. Carbon nanotubes have been successfully used in conjunction with bioconjugation to link TGF-β followed by an activation with near-infrared light. Typically, these reactions have involved the use of a crosslinker, but some of these add molecular space between the compound of interest and base material and in turn causes higher degrees of non-specific binding and unwanted reactivity.See also
*Immunofluorescence
Immunofluorescence (IF) is a light microscopy-based technique that allows detection and localization of a wide variety of target biomolecules within a cell or tissue at a quantitative level. The technique utilizes the binding specificity of anti ...
* Biomolecular engineering Biomolecular engineering is the application of engineering principles and practices to the purposeful manipulation of molecules of biological origin. Biomolecular engineers integrate knowledge of biological processes with the core knowledge of chemi ...
* Biotinylation
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of bi ...
* SpyTag/SpyCatcher
* In situ cyclization of proteins
* Unnatural amino acids
* '' Bioconjugate Chemistry'' journal
References
{{reflist, 30em Biochemistry Chemical bonding