cadmium iodide on:
[Wikipedia]
[Google]
[Amazon]
Cadmium iodide is an inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its
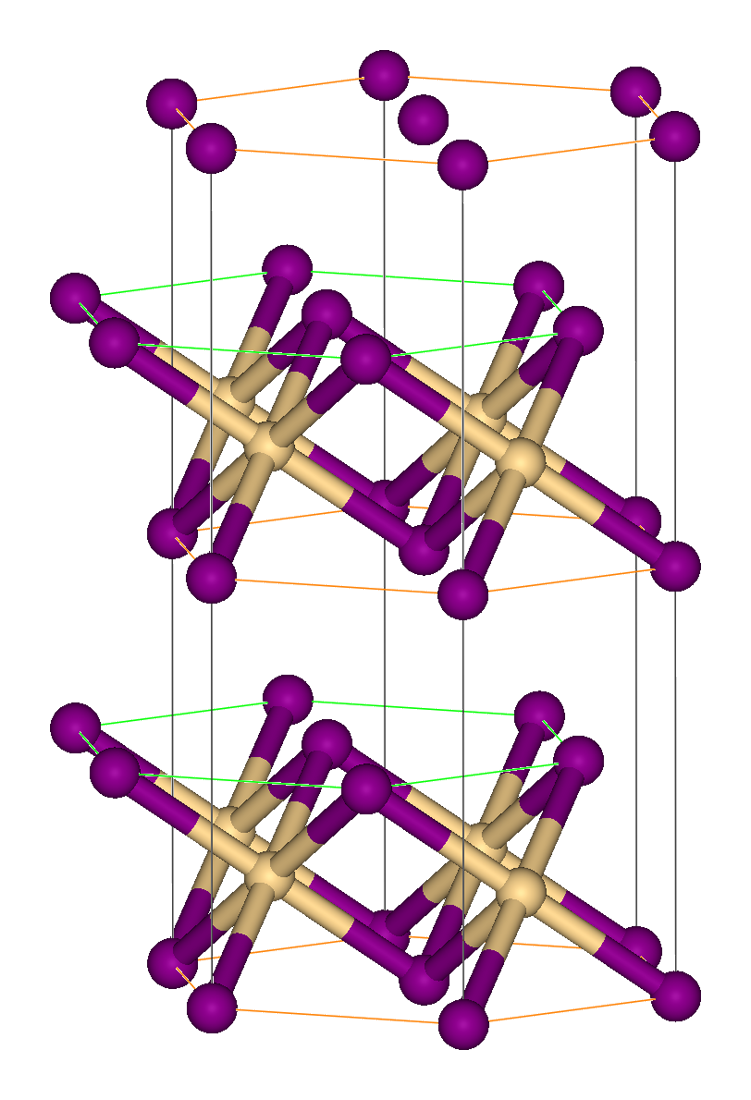 In cadmium iodide the iodide
In cadmium iodide the iodide
crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
, which is typical for compounds of the form MX2 with strong polarization effects.
Preparation
Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine.Applications
Historically, cadmium iodide was used as acatalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
for the Henkel process, a high-temperature isomerisation of dipotassium phthalate to yield the terephthalate. The salt was then treated with acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
to yield potassium acetate and commercially valuable terephthalic acid.
While uneconomical compared to the production of terephthalic acid from ''p''-xylene, the Henkel method has been proposed as a potential route to produce terephthalic acid from furfural. As existing Bio-PET is still reliant on petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
as a source of ''p''-xylene, the Henkel process could theoretically offer a completely bioplastic
Bioplastics are plastic materials produced from renewable biomass sources. Timeline of plastic development, Historically, bioplastics made from natural materials like shellac or Celluloid, cellulose had been the first plastics. Since the end of ...
route to polyethylene terephthalate
Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in synthetic fibre, fibres for clothing, packaging, conta ...
.
Crystal structure
 In cadmium iodide the iodide
In cadmium iodide the iodide anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s form a hexagonal closely packed arrangement while the cadmium cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s fill all of the octahedral sites in alternate layers. The resultant structure consists of a layered lattice. This same basic structure is found in many other salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
s and mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Mi ...
s. Cadmium iodide is mostly ionically bonded but with partial covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
character.
Cadmium iodide's crystal structure is the prototype on which the crystal structures of many other compounds can be considered to be based. Compounds with any of the following characteristics tend to adopt the CdI2 structure:
* Iodides of moderately polarising cations; bromides and chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
s of strongly polarising cations
* Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It ...
s of dications, i.e. compounds with the general formula M(OH)2
* Sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
s, selenides and tellurides (chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the rad ...
ides) of tetracations, i.e. compounds with the general formula MX2, where X = S, Se, Te
References
{{Iodides Cadmium compounds Iodides Metal halides Photographic chemicals Crystal structure types