Borrowing Hydrogen on:
[Wikipedia]
[Google]
[Amazon]

 Hydrogen auto-transfer, also known as borrowing hydrogen, is the
Hydrogen auto-transfer, also known as borrowing hydrogen, is the
 The formation of carbon–carbon bond has been achieved through borrowing hydrogen-type Indirect witting, aldol, Knoevenagel condensation and also through various carbon nucleophiles. Related to the Guerbet reaction, Donohoe and coworkers have developed enantioselective borrowing hydrogen-type enolate alkylations.
The formation of carbon–carbon bond has been achieved through borrowing hydrogen-type Indirect witting, aldol, Knoevenagel condensation and also through various carbon nucleophiles. Related to the Guerbet reaction, Donohoe and coworkers have developed enantioselective borrowing hydrogen-type enolate alkylations.
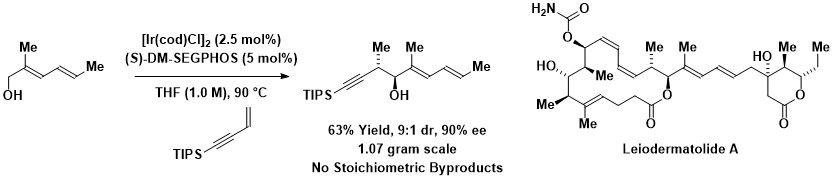
 In 2008, borrowing hydrogen reactions of 1,3-enynes with alcohols to form products of carbonyl propargylation was discovered. An enantioselective variant of this method was recently used in the total synthesis of leiodermatolide A.
In 2008, borrowing hydrogen reactions of 1,3-enynes with alcohols to form products of carbonyl propargylation was discovered. An enantioselective variant of this method was recently used in the total synthesis of leiodermatolide A.
activation
Activation, in chemistry and biology, is the process whereby something is prepared or excited for a subsequent reaction.
Chemistry
In chemistry, "activation" refers to the reversible transition of a molecule into a nearly identical chemical ...
of a chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and break ...
by temporary transfer of two hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen cons ...
s from the reactant to a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
and return of those hydrogen atoms back to a reaction intermediate
In chemistry, a reaction intermediate or an intermediate is a molecular entity that is formed from the reactants (or preceding intermediates) but is consumed in further reactions in stepwise chemical reactions that contain multiple elementary ...
to form the final product
Product may refer to:
Business
* Product (business), an item that serves as a solution to a specific consumer problem.
* Product (project management), a deliverable or set of deliverables that contribute to a business solution
Mathematics
* Prod ...
. Two major classes of borrowing hydrogen reactions exist: (a) those that result in hydroxyl substitution, and (b) those that result in carbonyl addition. In the former case, alcohol dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. A ...
generates a transient carbonyl compound that is subject to condensation followed by the return of hydrogen. In the latter case, alcohol dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. A ...
is followed by reductive generation of a nucleophile, which triggers carbonyl addition. As borrowing hydrogen processes avoid manipulations otherwise required for discrete alcohol oxidation and the use of stoichiometric organometallic reagents, they typically display high levels of atom-economy and, hence, are viewed as examples of Green chemistry
Green chemistry, also called sustainable chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. While environmental ch ...
.
History
The Guerbet reaction, reported in 1899, is an early example of a hydrogen auto-transfer process. The Guerbet reaction converts primary alcohols to β-alkylated dimers via alcohol dehydrogenation followed by aldol condensation and reduction of the resulting enones. Application of the Guerbet reaction to the development of ethanol-to-butanol processes has garnered interest as a method for the production ofrenewable fuels
Renewable Fuels are fuels produced from renewable resources. Examples include: biofuels (e.g. Vegetable oil used as fuel, ethanol, methanol from clean energy and carbon dioxide or biomass, and biodiesel) and Hydrogen fuel (when produced with ...
. In 1932 using heterogeneous nickel-catalysts Adkins reported the first alcohol aminations that occur through alcohol dehydrogenation-reductive amination. Homogenous catalysts for alcohol amination based on rhodium and ruthenium were developed by Grigg and Watanabe in 1981. The first hydrogen auto-transfer processes that convert primary alcohols to products of carbonyl addition were reported by Michael J. Krische
Michael J. Krische (Fellow of the Royal Society of Chemistry, FRSC, Fellow of the American Association for the Advancement of Science, FAAAS; born September 16, 1966) is an American chemist and Robert A. Welch Chair in Science at the Department of ...
in 2007-2008 using homogenous iridium and ruthenium catalysts.
Hydroxyl substitution
Alcohol aminations are among the most commonly utilized borrowing hydrogen processes. In reactions of this type, alcohol dehydrogenation is followed by reductive amination of the resulting carbonyl compound. This represents an alternative to two-step processes involving conversion of the alcohol to a halide or sulfonate ester followed by nucleophilic substitution As shown below, alcohol amination has been used on kilogram scale byPfizer
Pfizer Inc. ( ) is an American multinational pharmaceutical and biotechnology corporation headquartered on 42nd Street in Manhattan, New York City. The company was established in 1849 in New York by two German entrepreneurs, Charles Pfize ...
for the synthesis of advanced pharmaceutical intermediates. Additionally, AstraZeneca
AstraZeneca plc () is a British-Swedish multinational pharmaceutical and biotechnology company with its headquarters at the Cambridge Biomedical Campus in Cambridge, England. It has a portfolio of products for major diseases in areas includ ...
has used methanol as an alternative to conventional genotoxic methylating agents such as methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one ...
or dimethyl sulfate
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as ( CH3)2 SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agen ...
. Nitroaromatics can also participate as amine precursors in borrowing hydrogen-type alcohol aminations.
 The formation of carbon–carbon bond has been achieved through borrowing hydrogen-type Indirect witting, aldol, Knoevenagel condensation and also through various carbon nucleophiles. Related to the Guerbet reaction, Donohoe and coworkers have developed enantioselective borrowing hydrogen-type enolate alkylations.
The formation of carbon–carbon bond has been achieved through borrowing hydrogen-type Indirect witting, aldol, Knoevenagel condensation and also through various carbon nucleophiles. Related to the Guerbet reaction, Donohoe and coworkers have developed enantioselective borrowing hydrogen-type enolate alkylations.
Carbonyl addition
As exemplified by the Krische allylation, dehydrogenation of alcohol reactants can be balanced by reduction of allenes, dienes or allyl acetate to generate allylmetal-carbonyl pairs that combine to give products of carbonyl addition. In this way, lower alcohols are directly transformed to higher alcohols in a manner that significantly decreases waste. In 2008, borrowing hydrogen reactions of 1,3-enynes with alcohols to form products of carbonyl propargylation was discovered. An enantioselective variant of this method was recently used in the total synthesis of leiodermatolide A.
In 2008, borrowing hydrogen reactions of 1,3-enynes with alcohols to form products of carbonyl propargylation was discovered. An enantioselective variant of this method was recently used in the total synthesis of leiodermatolide A.

References
{{reflist Catalysis