Bornyl Diphosphate Synthase on:
[Wikipedia]
[Google]
[Amazon]
In
 As seen in the mechanism above, bornyl diphosphate synthase catalyzes the cyclization cascade of GPP into (+)- bornyl diphosphate. Following the initial metal-activated diphosphate departure from GPP, the molecule isomerizes to linalyl diphosphate (LPP), which then allows for the rotation around the carbon-carbon bond, and consequent reattachment of the PPi group. The pyrophosphate then stabilizes the cyclization into the terpinyl cation, and another final cyclization yields the 2-bornyl cation. This cation is then neutralized by the stereo-specific C–O bond formation with the final re-attachment of pyrophosphate to create the final product, BPP. Careful consideration of the BPPS structure shows that the active site, discussed in further detail below, guides the positions and conformations of the isoprenoid functionality of the substrate, while the diphosphate position remains essentially anchored in a single location and conformation. Overall, the pyrophosphate plays an important role in stabilizing the carbocations formed throughout the cyclization in the active site of the enzyme. These interactions and the strategic positioning of pyrophosphate is what is believed to lead to its endo-specific recapture in the final step by the bornyl cation.
As seen in the mechanism above, bornyl diphosphate synthase catalyzes the cyclization cascade of GPP into (+)- bornyl diphosphate. Following the initial metal-activated diphosphate departure from GPP, the molecule isomerizes to linalyl diphosphate (LPP), which then allows for the rotation around the carbon-carbon bond, and consequent reattachment of the PPi group. The pyrophosphate then stabilizes the cyclization into the terpinyl cation, and another final cyclization yields the 2-bornyl cation. This cation is then neutralized by the stereo-specific C–O bond formation with the final re-attachment of pyrophosphate to create the final product, BPP. Careful consideration of the BPPS structure shows that the active site, discussed in further detail below, guides the positions and conformations of the isoprenoid functionality of the substrate, while the diphosphate position remains essentially anchored in a single location and conformation. Overall, the pyrophosphate plays an important role in stabilizing the carbocations formed throughout the cyclization in the active site of the enzyme. These interactions and the strategic positioning of pyrophosphate is what is believed to lead to its endo-specific recapture in the final step by the bornyl cation.
 Bornyl diphosphate synthase is a homodimeric isomerase, with each of the two monomers containing two α-helical domains. In the case of BPPS, the C-terminal domain directly catalyzes the cyclization of geranyl diphosphate, as seen in the above reaction mechanism, while the N-terminal domain acts as a scaffolding to the active site of the C-terminal during the reaction. The N-terminal domain forms similar α-barrels to that of other terpene cyclases such as epiaristolochene synthase and
Bornyl diphosphate synthase is a homodimeric isomerase, with each of the two monomers containing two α-helical domains. In the case of BPPS, the C-terminal domain directly catalyzes the cyclization of geranyl diphosphate, as seen in the above reaction mechanism, while the N-terminal domain acts as a scaffolding to the active site of the C-terminal during the reaction. The N-terminal domain forms similar α-barrels to that of other terpene cyclases such as epiaristolochene synthase and
enzymology
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
, bornyl diphosphate synthase (BPPS) () is an enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
that catalyzes
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
the chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
:geranyl diphosphate (+)-bornyl diphosphate
Bornyl diphosphate synthase is involved in the biosynthesis of the cyclic monoterpenoid
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen funct ...
bornyl diphosphate. As seen from the reaction above, BPPS takes geranyl diphosphate
Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpenoid geraniol. Its salts are colorless. It is a precursor to many thousands of natural products.
Occurrence
GPP is an intermediate in the ...
as its only substrate and isomerizes into the product, (+)- bornyl diphosphate. This reaction comes from a general class of enzymes called terpene synthases that cyclize a universal precursor, geranyl diphosphate, to form varying monocyclic and bicyclic monoterpenes. The biochemical transformation of geranyl diphosphate to cyclic products occurs in a variety of aromatic plants, including both angiosperms
Flowering plants are plants that bear flowers and fruits, and form the clade Angiospermae (). The term angiosperm is derived from the Greek words (; 'container, vessel') and (; 'seed'), meaning that the seeds are enclosed within a fruit. T ...
and gymnosperm
The gymnosperms ( ; ) are a group of woody, perennial Seed plant, seed-producing plants, typically lacking the protective outer covering which surrounds the seeds in flowering plants, that include Pinophyta, conifers, cycads, Ginkgo, and gnetoph ...
s, and is used for various purposes described in sections below. Terpene synthases like BPPS are the primary enzymes in the formation of low-molecular-weight terpene metabolites. The organization of terpene synthases, their characteristic ability to form multiple products, and regulation in response to biotic and abiotic factors contribute to the formation of a diverse group of terpene metabolites. The structural diversity and complexity of terpenes generates an enormous potential for mediating plant–environment interactions.
The systematic name
A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature.
A semisystematic name or semitrivi ...
of this enzyme class is (+)-bornyl-diphosphate lyase (decyclizing). Other names in common use include bornyl pyrophosphate synthase, bornyl pyrophosphate synthetase, (+)-bornylpyrophosphate cyclase, and geranyl-diphosphate cyclase (ambiguous). This enzyme participates in monoterpenoid
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen funct ...
biosynthesis and belongs to the family of isomerase
In biochemistry, isomerases are a general class of enzymes that convert a molecule from one isomer to another. Isomerases facilitate intramolecular rearrangements in which chemical bond, bonds are Bond cleavage, broken and formed. The general form ...
s, specifically the class of intramolecular lyases.
The chirality of borneol in a plant depends on the preferred chirality of the bornyl diphosphate synthase. Synthases for either chirality have been sequenced: this article deals with the better-characterized (+)-bornyl diphosphate synthase. In 2021, scientists discovered the first known (-)-bornyl diphosphate synthase.
Mechanism
 As seen in the mechanism above, bornyl diphosphate synthase catalyzes the cyclization cascade of GPP into (+)- bornyl diphosphate. Following the initial metal-activated diphosphate departure from GPP, the molecule isomerizes to linalyl diphosphate (LPP), which then allows for the rotation around the carbon-carbon bond, and consequent reattachment of the PPi group. The pyrophosphate then stabilizes the cyclization into the terpinyl cation, and another final cyclization yields the 2-bornyl cation. This cation is then neutralized by the stereo-specific C–O bond formation with the final re-attachment of pyrophosphate to create the final product, BPP. Careful consideration of the BPPS structure shows that the active site, discussed in further detail below, guides the positions and conformations of the isoprenoid functionality of the substrate, while the diphosphate position remains essentially anchored in a single location and conformation. Overall, the pyrophosphate plays an important role in stabilizing the carbocations formed throughout the cyclization in the active site of the enzyme. These interactions and the strategic positioning of pyrophosphate is what is believed to lead to its endo-specific recapture in the final step by the bornyl cation.
As seen in the mechanism above, bornyl diphosphate synthase catalyzes the cyclization cascade of GPP into (+)- bornyl diphosphate. Following the initial metal-activated diphosphate departure from GPP, the molecule isomerizes to linalyl diphosphate (LPP), which then allows for the rotation around the carbon-carbon bond, and consequent reattachment of the PPi group. The pyrophosphate then stabilizes the cyclization into the terpinyl cation, and another final cyclization yields the 2-bornyl cation. This cation is then neutralized by the stereo-specific C–O bond formation with the final re-attachment of pyrophosphate to create the final product, BPP. Careful consideration of the BPPS structure shows that the active site, discussed in further detail below, guides the positions and conformations of the isoprenoid functionality of the substrate, while the diphosphate position remains essentially anchored in a single location and conformation. Overall, the pyrophosphate plays an important role in stabilizing the carbocations formed throughout the cyclization in the active site of the enzyme. These interactions and the strategic positioning of pyrophosphate is what is believed to lead to its endo-specific recapture in the final step by the bornyl cation.
Enzyme Structure
 Bornyl diphosphate synthase is a homodimeric isomerase, with each of the two monomers containing two α-helical domains. In the case of BPPS, the C-terminal domain directly catalyzes the cyclization of geranyl diphosphate, as seen in the above reaction mechanism, while the N-terminal domain acts as a scaffolding to the active site of the C-terminal during the reaction. The N-terminal domain forms similar α-barrels to that of other terpene cyclases such as epiaristolochene synthase and
Bornyl diphosphate synthase is a homodimeric isomerase, with each of the two monomers containing two α-helical domains. In the case of BPPS, the C-terminal domain directly catalyzes the cyclization of geranyl diphosphate, as seen in the above reaction mechanism, while the N-terminal domain acts as a scaffolding to the active site of the C-terminal during the reaction. The N-terminal domain forms similar α-barrels to that of other terpene cyclases such as epiaristolochene synthase and farnesyltransferase
Farnesyltransferase () is one of the three enzymes in the prenyltransferase group. Farnesyltransferase (FTase) adds a 15-carbon isoprenoid called a farnesyl group to proteins bearing a CaaX motif: a four-amino acid sequence at the carboxyl ...
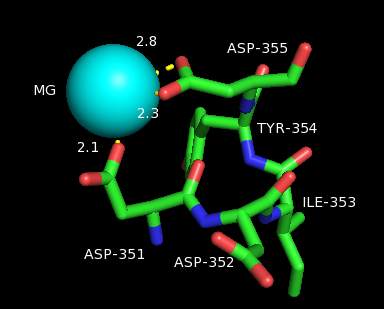
. In ligand complexes, such as with GPP, bornyl diphosphate synthase stabilizes the complex through multiple hydrogen bond interactions, specifically with aspartate-rich motifs. Additionally, arginines in the N terminus may play a stabilizing role in the initial isomerization step of the reaction cascade discussed in the section above. The C-terminal domain, on the other hand, contains 12 α-helices, which define the hydrophobic active site where the cyclization occurs. Critical amino acid segments found in the C-terminal domain are also what allow the required magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
metal ions to bind and allow the first pyrophosphate release. Specifically, this is accomplished by an aspartate-rich domain DDIYD beginning at D351, in with the boldface represents the residues directly interacting with the magnesium ion, elucidated on the adjacent image.
As of late 2007, 7 structures
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
have been solved for this class of enzymes, with PDB accession codes , , , , , , and .
Biological Function
Many properties of plants derive almost exclusively frommonoterpene
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen func ...
natural products: plants generate these compounds for molecular functions in regulation, communication, and defense. For examples, terpenes often have a strong odor and may protect the plants that produce them from herbivores by deterring them and by attracting predators of said herbivores. The monoterpenes characterized to-date reveal a vast array of structural and functional variations coming from different monocyclic or bicyclic skeletons. Despite this structural and stereochemical diversity, all monoterpenes derive from the same substrate, geranyl diphosphate
Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpenoid geraniol. Its salts are colorless. It is a precursor to many thousands of natural products.
Occurrence
GPP is an intermediate in the ...
(GPP). The cyclization of this C10-isoprenoid precursor through sequential carbocation intermediates, as seen in the above sections, and is catalyzed by metal-dependent enzymes: in this case, BPPS cyclizes GPP into bornyl diphosphate. However, the multitude of products coming from only a single substrate helps conclude that this diversity is a consequence of the evolution of variations in the enzyme. Each different enzyme holds an active site that chaperones intermediates through different cyclization pathways, and thus forms myriad monoterpenoids.
Industrial Relevance
Historically, aromatic plants have been used for their pleasing fragrances, culinary applications, and therapeutic potential. Because bornyl diphosphate synthase is crucial in forming aromatic monoterpenoids within plants, this enzyme is of key industrial relevance. Specifically, while most studies focus on BPPS from ''Salvia officinalis
''Salvia officinalis'', the common sage or sage, is a perennial, evergreen subshrub, with woody stems, grayish leaves, and blue to purplish flowers. It is a member of the mint family Lamiaceae and native to the Mediterranean region, though it ...
'', there has been a recent interest in studying LaBPPS, bornyl diphosphate synthase from lavender
''Lavandula'' (common name lavender) is a genus of 47 known species of perennial flowering plants in the sage family, Lamiaceae. It is native plant, native to the Old World, primarily found across the drier, warmer regions of the Mediterranean ...
. This interest arises from the fact that lavender essential oil
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the ...
s (EOs) of higher quality produced by a few ''Lavandula angustifolia
''Lavandula angustifolia'', formerly ''L. officinalis'', is a flowering plant in the family Lamiaceae, native to the Mediterranean (Spain, France, Italy, Croatia etc.). Its common names include lavender, true lavender and English lavender (though ...
'' variations are heavily sought after in the perfume industry. Compared to the BPPS of ''Salvia officinalis'', LaBPPS showed several differences in amino acid sequence, and the products it catalyzes: in detail, the carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
intermediates are more stable in LaBPPS than in regular BPPS, leading to a different efficiency of converting GPP into BPP. Given the novelty of LaBPP discovery, further research on this will most likely be of significant use to the perfume and fragrance industry.
References
* {{Portal bar, Biology, border=no EC 5.5.1 Enzymes of known structure