Alpha process on:
[Wikipedia]
[Google]
[Amazon]
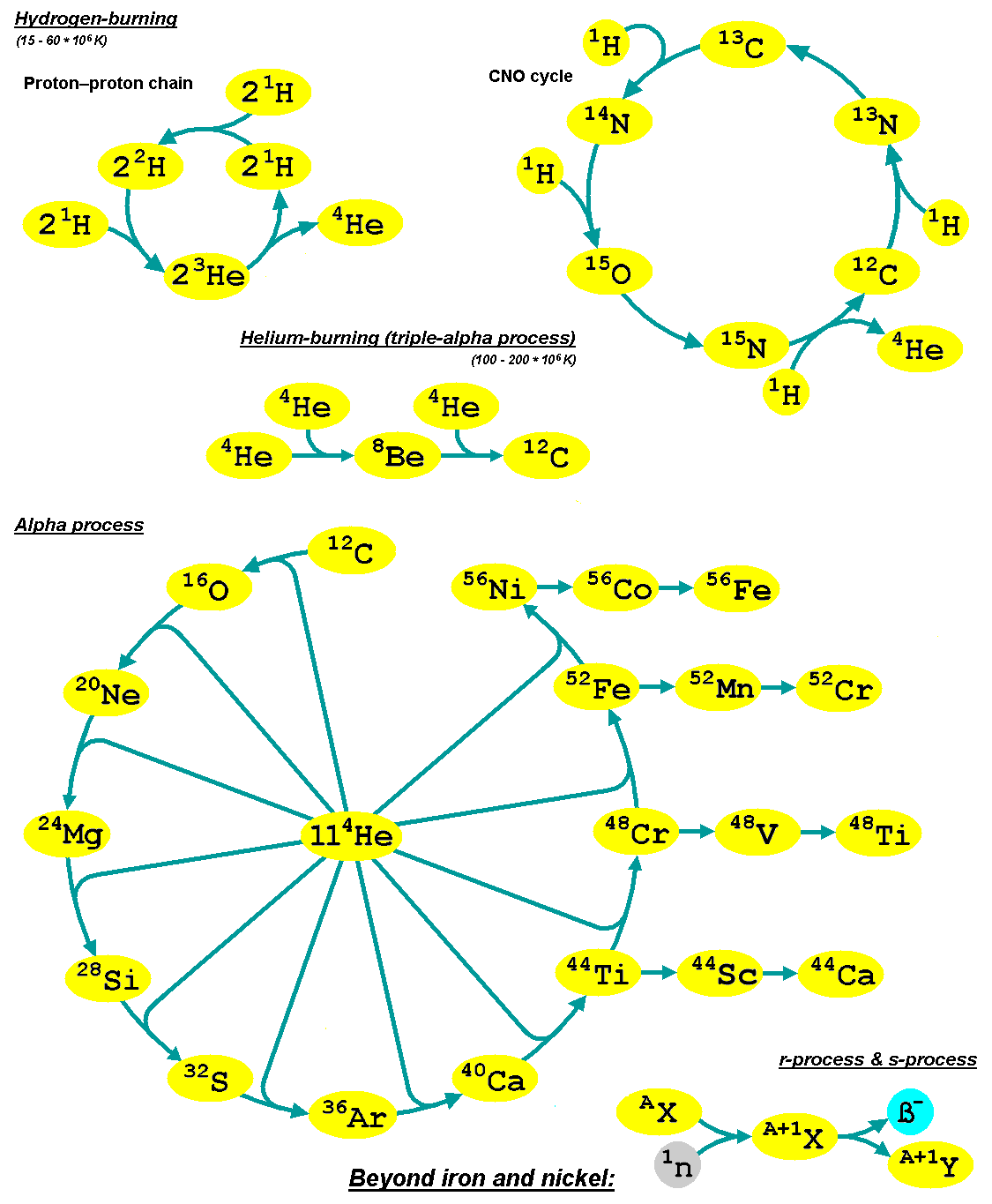 The alpha process, also known as the alpha ladder, is one of two classes of
The alpha process, also known as the alpha ladder, is one of two classes of
 * The stable alpha elements are: C, O, Ne, Mg, Si, and S.
* The elements Ar and Ca are ''"
* The stable alpha elements are: C, O, Ne, Mg, Si, and S.
* The elements Ar and Ca are ''"
 The alpha process, also known as the alpha ladder, is one of two classes of
The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion
Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles ( neutrons or protons). The difference in mass between the reactants and products is manife ...
reactions by which stars convert helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic ta ...
into heavier elements, the other being the triple-alpha process.
The triple-alpha process consumes only helium, and produces carbon. After enough carbon has accumulated, further reactions below take place, listed below. Each step only consumes helium and the product of the previous reaction.
:
The energy produced each the reaction, , is primarily in the gamma ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically ...
(), with a small amount taken by the byproduct element, as added momentum.
It is a common misconception that the above sequence ends at (or , which is a decay product of ) because it is the most tightly bound nuclide
A nuclide (or nucleide, from atomic nucleus, nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was co ...
- i.e., having the highest nuclear binding energy
Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is alway ...
per nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number (nucleon number).
Until the 1960s, nucleons were ...
, and production of heavier nuclei would require energy (be endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. ...
) instead of releasing it (exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity ...
). ( Nickel-62) is actually the most tightly bound nuclide in terms of binding energy (though Fe has a lower energy or mass per nucleon). The reaction is actually exothermic, but nonetheless the sequence does effectively end at iron. The sequence stops before producing because conditions in stellar interiors cause the competition between photodisintegration and the alpha process to favor photodisintegration around iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
. This leads to more being produced than
All these reactions have a very low rate at the temperatures and densities in stars and therefore do not contribute significant energy to a star's total output. They occur even less easily with elements heavier than neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypt ...
due to the increasing Coulomb barrier.
Alpha process elements
Alpha process elements (or alpha elements) are so-called since their most abundant isotopes are integer multiples of four – the mass of the helium nucleus (thealpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be prod ...
). These isotopes are called ''alpha nuclide
An alpha nuclide is a nuclide that consists of an integer number of alpha particles. Alpha nuclides have equal, even numbers of protons and neutrons; they are important in stellar nucleosynthesis since the energetic environment within stars is a ...
s''.
observationally stable
Stable nuclides are nuclides that are not radioactive and so (unlike radionuclides) do not spontaneously undergo radioactive decay. When such nuclides are referred to in relation to specific elements, they are usually termed stable isotopes.
Th ...
"''. They are synthesized by alpha capture prior to the silicon fusing stage, that leads to
* Si and Ca are purely alpha process elements.
* Mg can be separately consumed by proton capture
Proton capture is a nuclear reaction in which an atomic nucleus and one or more protons collide and merge to form a heavier nucleus.
Since protons have positive electric charge, they are repelled electrostatically by the positively charged nucl ...
reactions.
The status of oxygen ( O) is contested – some authors consider it an alpha element, while others do not. O is surely an alpha element in low-metallicity
In astronomy, metallicity is the abundance of elements present in an object that are heavier than hydrogen and helium. Most of the normal physical matter in the Universe is either hydrogen or helium, and astronomers use the word ''"metals"'' as ...
Population II stars: It is produced in Type II supernovas, and its enhancement is well correlated with an enhancement of other alpha process elements.
Sometimes C and N are considered alpha process elements since, like O, they are synthesized in nuclear alpha-capture reactions, but their status is ambiguous: Each of the three elements is produced (and consumed) by the CNO cycle
The CNO cycle (for carbon–nitrogen–oxygen; sometimes called Bethe–Weizsäcker cycle after Hans Albrecht Bethe and Carl Friedrich von Weizsäcker) is one of the two known sets of fusion reactions by which stars convert hydrogen to helium, ...
, which can proceed at temperatures far lower than those where the alpha process starts producing significant amounts of alpha elements (including C, N, & O). So just the presence of C, N, or O in a star does not a clearly indicate that the alpha process is actually underway – hence reluctance of some astronomers to (unconditionally) call these three "alpha elements".
Special notation for relative abundance
The abundance of total alpha elements in stars is usually expressed in a logarithmic manner, with a square bracket notation: : where is the number of alpha elements per unit volume, and is the number of iron nuclei per unit volume. It is for the purpose of calculating the number that which elements are to be considered "alpha elements" becomes contentious. Theoreticalgalactic evolution
The study of galaxy formation and evolution is concerned with the processes that formed a heterogeneous universe from a homogeneous beginning, the formation of the first galaxies, the way galaxies change over time, and the processes that have gen ...
models predict that early in the universe there were more alpha elements relative to iron. Type II supernovae mainly synthesize oxygen and the alpha-elements ( Ne, Mg, Si, S, Ar, Ca, and Ti) while Type Ia supernova
A Type Ia supernova (read: "type one-A") is a type of supernova that occurs in binary systems (two stars orbiting one another) in which one of the stars is a white dwarf. The other star can be anything from a giant star to an even smaller white ...
e mainly produce elements of the iron peak ( Ti, V, Cr, Mn, Fe, Co, and Ni), but also alpha-elements.
References
External links
* {{Star Nuclear fusion Nucleosynthesis