Azide Complex on:
[Wikipedia]
[Google]
[Amazon]
 Transition metal azide complexes are
Transition metal azide complexes are
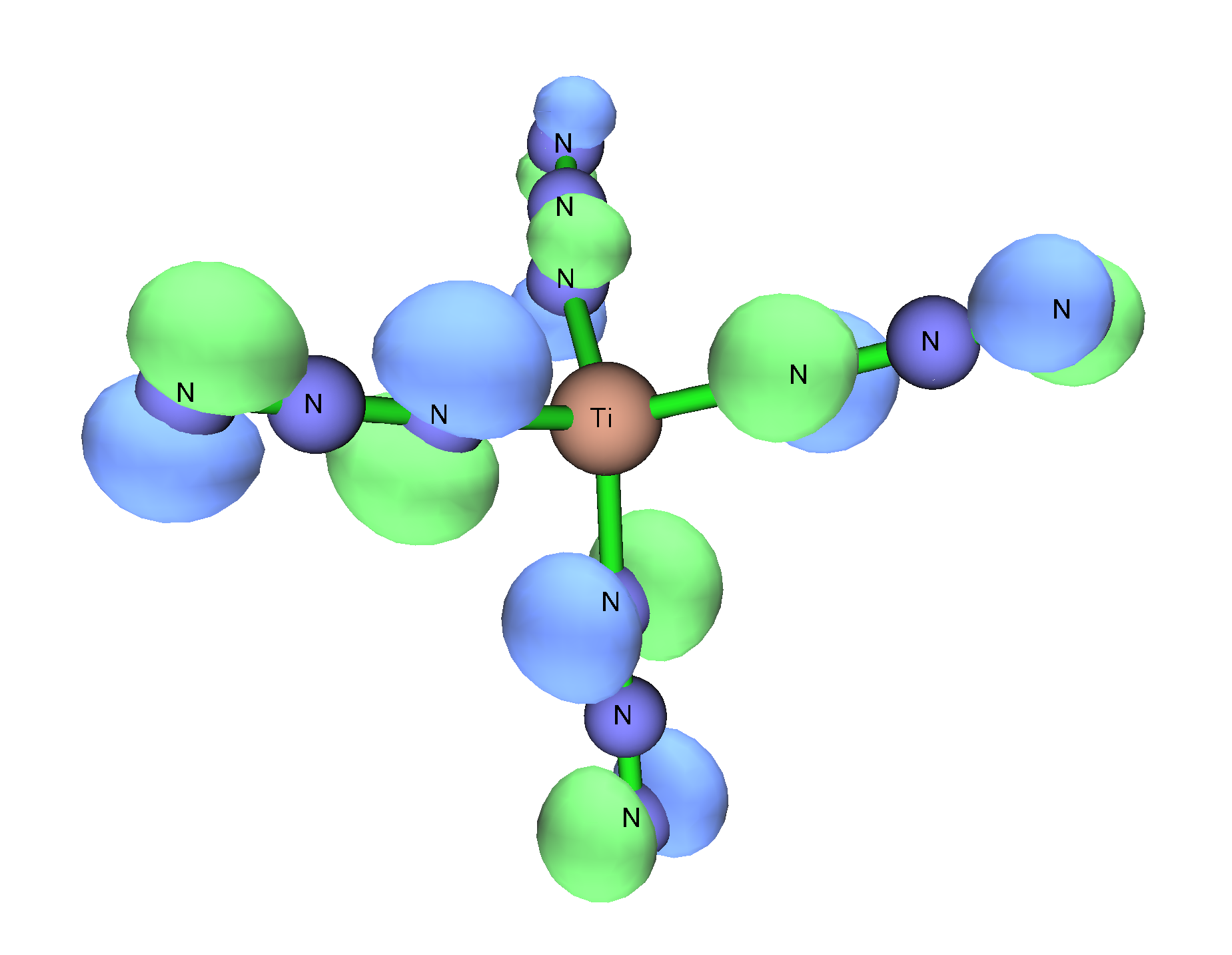 The main hypothesis given for why these compounds do not have linear M-N-N angles despite theoretical calculations is that these adducts are not tetrahedral. In the homoleptic
The main hypothesis given for why these compounds do not have linear M-N-N angles despite theoretical calculations is that these adducts are not tetrahedral. In the homoleptic
 The first Mn polyazide compound was prepared by Wöhler et al. in 1917 by reaction of MnCO3 with HN3 to form Mn(N3)2. Many divalent Mn azide salts have been synthesized. 1D chains are formed when 2,2’-bipyridine, a bidentate ligand, is used as the counter ion in the reaction between Mn(ClO4)2 • 6H2O and excess NaN3. This results in a chain with alternating EE and EO bridges which predictably gives alternating antiferromagnetic-ferromagnetic coupling. Another 2D structure is accessed via the reaction of (PPh4)2MnCl2 with AgN3 to form the Ph4sub>2 n(N3)4
The first example of a 3D azido compound was (CH3)4Mn(N3)3]. This compound has a pseudo- Perovskite (structure), perovskite structure with (CH3)4sup>+ ions in the cavities between the Mn centers. The azido moieties are arranged in an EE fashion, and indeed, this compound exhibits the expected antiferromagnetic behavior. The cesium analogue Cs n(N3)3is synthesized in a similar manner. For each 6 coordinate Mn, four of the azido linkages are EE and two are EO instead of all six being EE. This arrangement results in a honeycomb-like shape and a rare example of alternating ferro-antiferromagnetic interactions in 3D solid.
Examples of manganese azido compounds in higher oxidation states are rare. The triazide
The first Mn polyazide compound was prepared by Wöhler et al. in 1917 by reaction of MnCO3 with HN3 to form Mn(N3)2. Many divalent Mn azide salts have been synthesized. 1D chains are formed when 2,2’-bipyridine, a bidentate ligand, is used as the counter ion in the reaction between Mn(ClO4)2 • 6H2O and excess NaN3. This results in a chain with alternating EE and EO bridges which predictably gives alternating antiferromagnetic-ferromagnetic coupling. Another 2D structure is accessed via the reaction of (PPh4)2MnCl2 with AgN3 to form the Ph4sub>2 n(N3)4
The first example of a 3D azido compound was (CH3)4Mn(N3)3]. This compound has a pseudo- Perovskite (structure), perovskite structure with (CH3)4sup>+ ions in the cavities between the Mn centers. The azido moieties are arranged in an EE fashion, and indeed, this compound exhibits the expected antiferromagnetic behavior. The cesium analogue Cs n(N3)3is synthesized in a similar manner. For each 6 coordinate Mn, four of the azido linkages are EE and two are EO instead of all six being EE. This arrangement results in a honeycomb-like shape and a rare example of alternating ferro-antiferromagnetic interactions in 3D solid.
Examples of manganese azido compounds in higher oxidation states are rare. The triazide
 Pentaazidoiron (III) ion e(N3)5sup>2- can be made by treating iron(III) salts with sodium azide. An iron azide reagent can be generated ''in situ''. NaN3 and iron (III) sulfate Fe2(SO4)3 are combined in methanol and added to an
Pentaazidoiron (III) ion e(N3)5sup>2- can be made by treating iron(III) salts with sodium azide. An iron azide reagent can be generated ''in situ''. NaN3 and iron (III) sulfate Fe2(SO4)3 are combined in methanol and added to an
 Transition metal azide complexes are
Transition metal azide complexes are coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
es containing one or more azide
In chemistry, azide (, ) is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
(N3−) ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s. In addition to coordination complexes, this article summarizes homoleptic transition metal azides, which are often coordination polymer
Coordination may refer to:
* Coordination (linguistics), a compound grammatical construction
* Coordination complex, consisting of a central atom or ion and a surrounding array of bound molecules or ions
** A chemical reaction to form a coordinat ...
s.
Structure and bonding
Azide is a pseudohalide but more nucleophilic than chloride, as reflected by the higher pKa ofhydrazoic acid
Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, This also contains a detailed description of the contemporaneous production process. is a compound with the chemical formula . It is a colorless, volatile, and explosive liquid ...
(4.6) vs hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
(-5.9). As a monodentate ligand, azide binds through one of the two terminal nitrogen atoms, i.e. M-N=N=N. Azide is a "pure" sigma donor. It is classified as an X ligand in the Covalent bond classification method. In the usual electron counting method, it is a one-electron ligand.
The N3 unit is linear or nearly so. The M-N-N angles are quite bent. Azide functions as a bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually r ...
via two bonding modes. Commonly the metals share the same nitrogen ("N-diazonium" mode). Less common is the motif M-N=N=N-M, illustrated by u(N3)(PPh3)2sub>2.
General synthetic methods
Traditionally, metal azide complexes are prepared bysalt metathesis
A salt metathesis reaction (also called a double displacement reaction, double replacement reaction, or double decomposition) is a type of chemical reaction in which two ionic compounds in aqueous solution exchange their component ions to form two ...
, e.g. the reaction of metal chlorides with sodium azide
Sodium azide is an inorganic compound with the formula . This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is highly soluble in water and is acutely poisonou ...
. In some cases, trimethylsilyl azide
Trimethylsilyl azide is the organosilicon compound with the formula . A colorless liquid, it is a reagent in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, pr ...
is employed as the azide source. Another popular route include acid-base reactions hydrazoic acid
Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, This also contains a detailed description of the contemporaneous production process. is a compound with the chemical formula . It is a colorless, volatile, and explosive liquid ...
HN3 and either hydrido or lewis base complexes. Still other methods rely on halide-azide exchange with trimethylsilyl azide
Trimethylsilyl azide is the organosilicon compound with the formula . A colorless liquid, it is a reagent in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, pr ...
SiMe3N3 with the metal fluorides as incomplete halide/azide exchange is often seen when using the chloride derivatives.
Homoleptic complexes
Many homoleptic complexes (with only one kind of ligand) are known. Coordination numbers range from 2 (e.g., u(N3)2sup>−) to 7 (e.g., (N3)7sup>−). Many homoleptic complexes are octahedral anions of the type (N3)6sup>n-: *dianions for tetravalent metals V, Pt, Ti, Zr, Hf *trianions for trivalent metals Cr, Fe, Ru, Rh, Ir *tetraanions for the divalent Ni For some metals, homoleptic complexes exist in two oxidation states: u(N3)2sup>− vs u(N3)4sup>− and t(N3)6sup>2- vs t(N3)4sup>2-.Binary
Binary may refer to:
Science and technology Mathematics
* Binary number, a representation of numbers using only two values (0 and 1) for each digit
* Binary function, a function that takes two arguments
* Binary operation, a mathematical op ...
azide compounds can take on several structures including discrete compounds, or one- two, and three-dimensional nets, leading some to dub them as "polyazides". Reactivity studies of azide compounds are relatively limited due to how sensitive they can be.
Group 3
Neutral unsolvated group 3 polyazide is only known for divalent europium(II) compound, Eu(N3)2. Attempts to react lanthanide hydroxides with HN3 result in their basic azides, Ln(OH)(N3)2 or Ln(OH)2N3.Group 4
Group 4 polyazides of the formula M(N3)4 are predicted to have linear or near linear M-N-N angles unlike their main group counterparts which are predicted to have bent M-N-N angles. This couldn’t be proved in the case of Ti(N3)4, owing to difficulty in crystallization. However, incorporation of large spacer counterions or N-donor adducts makes the compounds far easier to work with. In the cases of Ph4sub>2 (N3)6(M=Ti, Zr, Hf), only the axialligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s exhibit near linear M-N-N angles whereas the equatorial ligands are closer to bent angles. This deviation in theory is also seen in the N-donor adducts.
 The main hypothesis given for why these compounds do not have linear M-N-N angles despite theoretical calculations is that these adducts are not tetrahedral. In the homoleptic
The main hypothesis given for why these compounds do not have linear M-N-N angles despite theoretical calculations is that these adducts are not tetrahedral. In the homoleptic tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
compounds, the nitrogen closest to the (+IV) metal center is positioned in such a way that the three valence electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
pairs can donate to the vacant d orbitals on the metal and therefore the azido can act as a tridentate donor ligand in which case the expected coordination would be linear. Since the adduct
In chemistry, an adduct (; alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is ...
compounds are not tetrahedral, the azido group can only act as a monodentate donor with two sterically active electron pairs which result in a bent M-N-N bond angles.
Group 5
The neutral binary V(IV) azide as well as V(III), V(IV), and V(V) azido ions are known. Similar to the neutral Ti(IV) azide, V(N3)4 is difficult to study due to high shock and temperature instability. However, (N3)6sup>2- paired with a large, inert counterion is relatively stable and crystalizeses as a near perfectoctahedral
In geometry, an octahedron (: octahedra or octahedrons) is any polyhedron with eight faces. One special case is the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex. Many types of i ...
. In contrast to V(IV), the neutral binary V(V) could not be synthesized and attempts result in the reduction of V(V) to V(IV) with the elimination of N2 gas. Fortunately, the oxidation potentials of anions are lower than that of their parent compounds so (N3)6sup>− can be formed. Unlike (N3)6sup>2-, (N3)6sup>− is highly shock sensitive and distorted from octahedral symmetry with three long and three short M-N bonds in ''mer'' positions.
The neutral binary Nb(N3)5 and Ta(N3)5 also exist, and the acetonitrile adducts of these compounds contain a nearly linear azido trans to the coordinating acetonitrile. They represent the first evidence of linear M-N-N bonding. The corresponding anions b(N3)6sup>−, b(N3)7sup>2-, a(N3)6sup>−, and a(N3)7sup>2- are known and accordingly are much less shock sensitive. The structure of the hexaazido monoanions are similar to other heptaazido monoanions with bent azido ligands despite being predicted to have perfect S6 symmetry in the gas phase for b(N3)6 The heptaazido dianions possess monocapped triangular-prismatic 1/4/2 structures unlike the actinide trianion (N3)7sup>3- which crystallizes as a monocapped octahedron or pentagonal bipyramid. Several N-donor adducts are known to exist as well. Reactions of the neutral binary NbF5 and TaF5 in the presence of Me3SiN3 with N-donors containing small bite angles such as 2,2’-bipyridine or 1,10-phenanthroline result in self ionization products of the type (N3)4L2sup>+ (N3)6sup>− (L= N-donor) whereas N-donors containing large bite angles such as 3,3’-bipryidine or 4,4’-bipyridine produces the neutral pentaazide adducts M(N3)5•L (L=N-donor).
Group 6
Both Mo(N3)6 and W(N3)6 have been synthesized, and W(N3)6 is stable enough to grow single crystals. Contrary to group 4 and group 5 binary azido compounds, the anionic o(N3)7sup>− and (N3)7sup>− are less stable and more sensitive to handle than their neutral parent compounds. Upon warming solutions of the heptaazido anions in either MeCN or SO2 to room temperature, the tetraazido nitrido ions Mo(N3)4sup>− and W(N3)4sup>− are formed with elimination of N2.Group 7
 The first Mn polyazide compound was prepared by Wöhler et al. in 1917 by reaction of MnCO3 with HN3 to form Mn(N3)2. Many divalent Mn azide salts have been synthesized. 1D chains are formed when 2,2’-bipyridine, a bidentate ligand, is used as the counter ion in the reaction between Mn(ClO4)2 • 6H2O and excess NaN3. This results in a chain with alternating EE and EO bridges which predictably gives alternating antiferromagnetic-ferromagnetic coupling. Another 2D structure is accessed via the reaction of (PPh4)2MnCl2 with AgN3 to form the Ph4sub>2 n(N3)4
The first example of a 3D azido compound was (CH3)4Mn(N3)3]. This compound has a pseudo- Perovskite (structure), perovskite structure with (CH3)4sup>+ ions in the cavities between the Mn centers. The azido moieties are arranged in an EE fashion, and indeed, this compound exhibits the expected antiferromagnetic behavior. The cesium analogue Cs n(N3)3is synthesized in a similar manner. For each 6 coordinate Mn, four of the azido linkages are EE and two are EO instead of all six being EE. This arrangement results in a honeycomb-like shape and a rare example of alternating ferro-antiferromagnetic interactions in 3D solid.
Examples of manganese azido compounds in higher oxidation states are rare. The triazide
The first Mn polyazide compound was prepared by Wöhler et al. in 1917 by reaction of MnCO3 with HN3 to form Mn(N3)2. Many divalent Mn azide salts have been synthesized. 1D chains are formed when 2,2’-bipyridine, a bidentate ligand, is used as the counter ion in the reaction between Mn(ClO4)2 • 6H2O and excess NaN3. This results in a chain with alternating EE and EO bridges which predictably gives alternating antiferromagnetic-ferromagnetic coupling. Another 2D structure is accessed via the reaction of (PPh4)2MnCl2 with AgN3 to form the Ph4sub>2 n(N3)4
The first example of a 3D azido compound was (CH3)4Mn(N3)3]. This compound has a pseudo- Perovskite (structure), perovskite structure with (CH3)4sup>+ ions in the cavities between the Mn centers. The azido moieties are arranged in an EE fashion, and indeed, this compound exhibits the expected antiferromagnetic behavior. The cesium analogue Cs n(N3)3is synthesized in a similar manner. For each 6 coordinate Mn, four of the azido linkages are EE and two are EO instead of all six being EE. This arrangement results in a honeycomb-like shape and a rare example of alternating ferro-antiferromagnetic interactions in 3D solid.
Examples of manganese azido compounds in higher oxidation states are rare. The triazide acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
adduct can be prepared using the fluoride exchange route to give Mn(N3)3CN as a dark red shock sensitive compound. Upon addition of PPh4N3 the compound disproportionates into an insensitive mixture of Ph4sub>2 n(N3)2and Ph4sub>2 n(N3)6 The Mn(IV) salt can be prepared on its own by using Cs2MnF6 as the starting material to give the highly explosive Cs2 n(N3)6
Group 8
 Pentaazidoiron (III) ion e(N3)5sup>2- can be made by treating iron(III) salts with sodium azide. An iron azide reagent can be generated ''in situ''. NaN3 and iron (III) sulfate Fe2(SO4)3 are combined in methanol and added to an
Pentaazidoiron (III) ion e(N3)5sup>2- can be made by treating iron(III) salts with sodium azide. An iron azide reagent can be generated ''in situ''. NaN3 and iron (III) sulfate Fe2(SO4)3 are combined in methanol and added to an organoborane
Organoboron chemistry or organoborane chemistry studies organoboron compounds, also called organoboranes. These chemical compounds combine boron and carbon; typically, they are organic derivatives of borane (BH3), as in the trialkyl boranes.
Or ...
followed by slow addition of 30% hydrogen peroxide, presumably forming Fe(N3)3. When combined with alkenes, the equivalent of hydrogen azide add in an anti-Markovnikov fashion.
-Bu4Nsub>3 u(N3)6is prepared by treating K2 uIVCl6with NaN3. N2 gas is liberated in this reaction, which involves reduction of Ru(IV) to Ru(III).
Group 9
Tetraazido cobalt(II) compounds have been isolated as both the tetraphenylphosphonium and tetraphenylarsonium salts from solutions of cobalt sulfate with a 15 time sexcess of NaN3 to yield h4Psub>2 o(N3)4and h4Assub>2 o(N3)4respectively. The autooxidation of solutions of o(N3)4sup>2- can be used as a colorimetric spot test for the presence ofsulfite
Sulfites or sulphites are compounds that contain the sulfite ion (systematic name: sulfate(IV) ion), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used.
Sulfites are ...
ions.
Tetrabutylammonium salts of rhodium(III) and iridium(III) azides are known and are prepared by reacting a large excess of NaN3 in an aqueous solution with the corresponding Na3 Cl6• 12H2O metal chloride salt to form -Bu4Nsub>3 h(N3)6and -Bu4Nsub>3 r(N3)6
Group 10
Thebinary
Binary may refer to:
Science and technology Mathematics
* Binary number, a representation of numbers using only two values (0 and 1) for each digit
* Binary function, a function that takes two arguments
* Binary operation, a mathematical op ...
nickel azide Ni(N3)2 has been prepared by distilling HN3 onto nickel carbonate. Samples of Ni(N3)2 decompose upon heating .
d(N3)4sup>2- anions are square planar
In chemistry, the square planar molecular geometry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the co ...
and the degree of interaction between the anion and its corresponding cation can be determined by the amount of deviation in the torsion angles from the ideal geometry. Various platinates t(N3)4sup>2- and t(N3)6sup>4- are known and are prepared from Pt chloride salts with NaN3. Pt(II) salts tend to be far less stable than the Pt(IV) versions, and they either decompose fairly rapidly upon standing or explode. Their sensitivity in part has been explained by poor crystal packing.
Group 11
Both copper(I) and copper(II) azides are known. The binary copper(I) azide, CuN3, which is white, is a one-dimensional polymer. Molecular Copper (II) azides include salts of u(N3)4sup>2- and u(N3)6sup>2-. n forms 1D chains wherein octahedral Cu(II) centers are linked by both EE and EO bridging azides. All copper azides are explosive but their sensitivities vary widely from the parent azides CuN3 and Cu(N3)2 which are extremely sensitive to the ions paired with large countercations that are practically insensitive. Silver (I) azide is a well known explosive compound and has been demonstrated to form a 2D coordination polymer with square planar Ag+ ions surrounded by azido ligands in an EE fashion. Slow ramping of temperature from 150 °C to 251 °C results in melting and slow decomposition but rapid heating to 300 °C results in an explosion. Gold(III) azide is known as the tetraethylammonium salt t4NAu(N3)4] and also adopts a square planar structure. However unlike the silver azide, the gold azide is not stable at room temperature and will decompose after a few days and its metal azide bonds have significant covalent character.Group 12
While Zinc azide, Zn(N3)2 has been known since the late 1890s, solvent free Zn(N3)2 was isolated for the first time in 2016 from a dry ethereal solution of HN3 and Et2Zn in ''n''-hexane. Zn(N3)2 crystallizes in three different polymorphs α-Zn(N3)2 and the labile β-Zn(N3)2 and γ-Zn(N3)2 forms. The first mercury (I) azide was realized by Curtius in 1890 by combining aqueous mercury(I) salts withalkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
azides and by combining HN3 with elemental mercury to produce Hg2(N3)2. Both mercury (I) and mercury(II) azides can be easily prepared by mixing the respective mercury nitrates with sodium azide in aqueous solution at roomtemperature. The mercury (II) azide Hg(N3)2 exists in two polymorphs α-Hg(N3)2 and β-Hg(N3)2. The β form is very labile
Lability refers to the degree that something is likely to undergo change. It is the opposite ( antonym) of stability.
Biochemistry
In reference to biochemistry, this is an important concept as far as kinetics is concerned in metalloprotein ...
and quickly turns into the α polymorphs at room temperature. However, the β polymorph can prepared in analogy to β-Pb(N3)2 by slow diffusion of aqueous NaN3 into a solution of Hg(NO3)2 separated by a layer of aqueous NaNO3, but crystals nearly always explode during formation leading to a mixture of α and β polymorphs.
Binary cadmium azide Cd(N3)2 can be prepared from CdCO3 and aqueous HN3. However, it is structural unrelated to the mercury or zinc anaolgues and is based on repeat units of Cd2(N3)10 double octahedrals.
Mixed ligand complexes
Azide forms myriad mixed ligand complexes. Examples include Zn(N3)2(NH3)2 and (C5H5)2Ti(N3)2.Reactions
A characteristic reaction of azide complexes and compounds) is degradation via loss of nitrogen gas. The stoichiometry for a diazide compound is: : The process often occurs explosively. Azide ligands are react with nitrosonium to givenitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or factitious air, among others, is a chemical compound, an Nitrogen oxide, oxide of nitrogen with the Chemical formula, formula . At room te ...
. This reaction is used to generate coordinatively unsaturated complexes.
: o(NH3)5N3sup>2+ + NO+ + H2O → o(NH3)5(H2O)sup>3+ + N2O + N2
This approach was used to prepare the previously elusive dicationic complex pentamminecobalt(III) perchlorate, .
See also
* Main group azido compoundsReferences