Atomic Radii on:
[Wikipedia]
[Google]
[Amazon]
 The atomic radius of a
The atomic radius of a  For many purposes, atoms can be modeled as spheres. This is only a crude approximation, but it can provide quantitative explanations and predictions for many phenomena, such as the
For many purposes, atoms can be modeled as spheres. This is only a crude approximation, but it can provide quantitative explanations and predictions for many phenomena, such as the
 Electrons in atoms fill
Electrons in atoms fill
La3+ > Ce3+ > ..., ... > Lu3+. # There is a regular decrease in their ionic radii. # There is a regular decrease in their tendency to act as a reducing agent, with an increase in atomic number. # The second and third rows of d-block transition elements are quite close in properties. # Consequently, these elements occur together in natural minerals and are difficult to separate.
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
is a measure of the size of its atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
, usually the mean or typical distance from the center of the nucleus
Nucleus (: nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucleu ...
to the outermost isolated electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. Four widely used definitions of atomic radius are: Van der Waals radius
The van der Waals radius, ''r'', of an atom is the radius of an imaginary hard sphere representing the distance of closest approach for another atom.
It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics ...
, ionic radius
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cati ...
, metallic radius
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be describ ...
and covalent radius
The covalent radius, ''r''cov, is a measure of the size of an atom that forms part of one covalent bond. It is usually measured either in picometres (pm) or angstroms (Å), with 1 Å = 100 pm.
In principle, the sum of the two cova ...
. Typically, because of the difficulty to isolate atoms in order to measure their radii separately, atomic radius is measured in a chemically bonded state; however theoretical calculations are simpler when considering atoms in isolation. The dependencies on environment, probe, and state lead to a multiplicity of definitions.
Depending on the definition, the term may apply to atoms in condensed matter
Condensed matter physics is the field of physics that deals with the macroscopic and microscopic physical properties of matter, especially the solid and liquid phases, that arise from electromagnetic forces between atoms and electrons. More gen ...
, covalently bonding in molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s, or in ionized
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
and excited state
In quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Add ...
s; and its value may be obtained through experimental measurements, or computed from theoretical models. The value of the radius may depend on the atom's state and context.
Electrons do not have definite orbits nor sharply defined ranges. Rather, their positions must be described as probability distribution
In probability theory and statistics, a probability distribution is a Function (mathematics), function that gives the probabilities of occurrence of possible events for an Experiment (probability theory), experiment. It is a mathematical descri ...
s that taper off gradually as one moves away from the nucleus, without a sharp cutoff; these are referred to as atomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
s or electron clouds. Moreover, in condensed matter and molecules, the electron clouds of the atoms usually overlap to some extent, and some of the electrons may roam over a large region encompassing two or more atoms.
Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm ( trillionths of a meter), or between 0.3 and 3 ångström
The angstrom (; ) is a unit of length equal to m; that is, one ten- billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814� ...
s. Therefore, the radius of an atom is more than 10,000 times the radius of its nucleus (1–10 fm),
and less than 1/1000 of the wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
of visible light
Light, visible light, or visible radiation is electromagnetic radiation that can be visual perception, perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400– ...
(400–700 nm).
 For many purposes, atoms can be modeled as spheres. This is only a crude approximation, but it can provide quantitative explanations and predictions for many phenomena, such as the
For many purposes, atoms can be modeled as spheres. This is only a crude approximation, but it can provide quantitative explanations and predictions for many phenomena, such as the density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be u ...
of liquids and solids, the diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
of fluids through molecular sieves
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
, the arrangement of atoms and ions in crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s, and the size and shape of molecules.
History
The first to estimate the radius of an atom was Johann Chrysostom Magnenus in 1646. He was atMass
Mass is an Intrinsic and extrinsic properties, intrinsic property of a physical body, body. It was traditionally believed to be related to the physical quantity, quantity of matter in a body, until the discovery of the atom and particle physi ...
and noticed the smell of incense permeating the church. He knew the size of the incense and estimated the size of the church. He presumed that he could detect the incense if one atom was in each nostril. He also presumed that the incense was distributed homogenously throughout the church. With these assumptions he was able to estimate the size of an atom to be about 10 to the power of −24 cubic metres. (The units he used have been converted to metric to make comparisons with later estimates easier.) Taking the cube root this gives an estimate of the atomic radius to be about 10 to the power of −8 metres. This is somewhat larger than current estimates but given the assumptions made in the calculation is very good. These calculations were published in his work ''Democritus reviviscens sive de atomis''.
The concept of atomic radius was preceded in the 19th century by the concept of atomic volume, a relative measure of how much space would on average an atom occupy in a given solid or liquid material. By the end of the century this term was also used in an absolute sense, as a molar volume
In chemistry and related fields, the molar volume, symbol ''V''m, or \tilde V of a substance is the ratio of the volume (''V'') occupied by a substance to the amount of substance (''n''), usually at a given temperature and pressure. It is also eq ...
divided by Avogadro constant
The Avogadro constant, commonly denoted or , is an SI defining constant with an exact value of when expressed in reciprocal moles.
It defines the ratio of the number of constituent particles to the amount of substance in a sample, where th ...
. Such a volume is different for different crystalline forms even of the same compound, but physicists used it for rough, order-of-magnitude estimates of the atomic size, getting 10−8–10−7 cm for copper.
The earliest estimates of the atomic size was made by opticians in the 1830s, particularly Cauchy
Baron Augustin-Louis Cauchy ( , , ; ; 21 August 1789 – 23 May 1857) was a French mathematician, engineer, and physicist. He was one of the first to rigorously state and prove the key theorems of calculus (thereby creating real a ...
, who developed models of light dispersion
Dispersion may refer to:
Economics and finance
*Dispersion (finance), a measure for the statistical distribution of portfolio returns
* Price dispersion, a variation in prices across sellers of the same item
*Wage dispersion, the amount of variat ...
assuming a lattice of connected "molecules". In 1857 Clausius
Rudolf Julius Emanuel Clausius (; 2 January 1822 – 24 August 1888) was a German physicist and mathematician and is considered one of the central founding fathers of the science of thermodynamics. By his restatement of Nicolas Léonard Sadi Ca ...
developed a gas-kinetic model which included the equation for mean free path
In physics, mean free path is the average distance over which a moving particle (such as an atom, a molecule, or a photon) travels before substantially changing its direction or energy (or, in a specific context, other properties), typically as a ...
. In the 1870s it was used to estimate gas molecule sizes, as well as an aforementioned comparison with visible light
Light, visible light, or visible radiation is electromagnetic radiation that can be perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400–700 nanometres (nm ...
wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
and an estimate from the thickness of soap bubble
A soap bubble (commonly referred to as simply a bubble) is an extremely thin soap film, film of soap or detergent and water enclosing air that forms a hollow sphere with an iridescent surface. Soap bubbles usually last for only a few seconds b ...
film at which its contractile force rapidly diminishes. By 1900, various estimates of mercury atom diameter averaged around 275±20 pm (modern estimates give 300±10 pm, see below).
In 1920, shortly after it had become possible to determine the sizes of atoms using X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
, it was suggested that all atoms of the same element have the same radii. However, in 1923, when more crystal data had become available, it was found that the approximation of an atom as a sphere does not necessarily hold when comparing the same atom in different crystal structures.
Definitions
Widely used definitions of atomic radius include: *Van der Waals radius
The van der Waals radius, ''r'', of an atom is the radius of an imaginary hard sphere representing the distance of closest approach for another atom.
It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics ...
: In the simplest definition, half the minimum distance between the nuclei of two atoms of the element that are not otherwise bound by covalent or metallic interactions.
The Van der Waals radius may be defined even for elements (such as metals) in which Van der Waals forces are dominated by other interactions. Because Van der Waals interactions arise through quantum fluctuations of the atomic polarisation, the polarisability (which can usually be measured or calculated more easily) may be used to define the Van der Waals radius indirectly.
* Ionic radius
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cati ...
: the nominal radius of the ions of an element in a specific ionization state, deduced from the spacing of atomic nuclei in crystalline salts that include that ion. In principle, the spacing between two adjacent oppositely charged ions (the length
Length is a measure of distance. In the International System of Quantities, length is a quantity with Dimension (physical quantity), dimension distance. In most systems of measurement a Base unit (measurement), base unit for length is chosen, ...
of the ionic bond
Ionic bonding is a type of chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic ...
between them) should equal the sum of their ionic radii.
* Covalent radius
The covalent radius, ''r''cov, is a measure of the size of an atom that forms part of one covalent bond. It is usually measured either in picometres (pm) or angstroms (Å), with 1 Å = 100 pm.
In principle, the sum of the two cova ...
: the nominal radius of the atoms of an element when covalently bound to other atoms, as deduced from the separation between the atomic nuclei in molecules. In principle, the distance between two atoms that are bound to each other in a molecule (the length of that covalent bond) should equal the sum of their covalent radii.
* Metallic radius
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be describ ...
: the nominal radius of atoms of an element when joined to other atoms by metallic bond
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be descr ...
s.
* Bohr radius
The Bohr radius () is a physical constant, approximately equal to the most probable distance between the nucleus and the electron in a hydrogen atom in its ground state. It is named after Niels Bohr, due to its role in the Bohr model of an at ...
: the radius of the lowest-energy electron orbit predicted by Bohr model
In atomic physics, the Bohr model or Rutherford–Bohr model was a model of the atom that incorporated some early quantum concepts. Developed from 1911 to 1918 by Niels Bohr and building on Ernest Rutherford's nuclear Rutherford model, model, i ...
of the atom (1913).
It is only applicable to atoms and ions with a single electron, such as hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, singly ionized helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
, and positronium
Positronium (Ps) is a system consisting of an electron and its antimatter, anti-particle, a positron, bound together into an exotic atom, specifically an onium. Unlike hydrogen, the system has no protons. The system is unstable: the two part ...
. Although the model itself is now obsolete, the Bohr radius for the hydrogen atom is still regarded as an important physical constant, because it is equivalent to the quantum-mechanical most probable distance of the electron from the nucleus.
Empirically measured atomic radius
The following table shows empirically measured covalent radii for the elements, as published by J. C. Slater in 1964. The values are in picometers (pm or 1×10−12 m), with an accuracy of about 5 pm. The shade of the box ranges from red to yellow as the radius increases; gray indicates lack of data.Explanation of the general trends
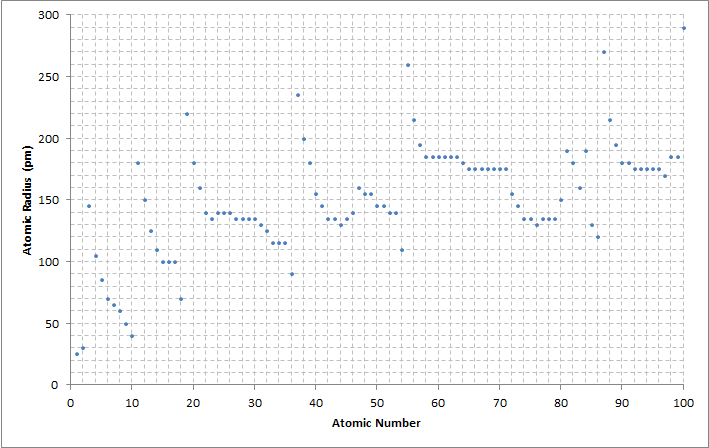
 Electrons in atoms fill
Electrons in atoms fill electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (o ...
s from the lowest available energy level. As a consequence of the Aufbau principle
In atomic physics and quantum chemistry, the Aufbau principle (, from ), also called the Aufbau rule, states that in the ground state of an atom or ion, electrons first fill Electron shell#Subshells, subshells of the lowest available energy, the ...
, each new period
Period may refer to:
Common uses
* Period (punctuation)
* Era, a length or span of time
*Menstruation, commonly referred to as a "period"
Arts, entertainment, and media
* Period (music), a concept in musical composition
* Periodic sentence (o ...
begins with the first two elements filling the next unoccupied s-orbital
In quantum mechanics, an atomic orbital () is a function describing the location and wave-like behavior of an electron in an atom. This function describes an electron's charge distribution around the atom's nucleus, and can be used to calc ...
. Because an atom's s-orbital electrons are typically farthest from the nucleus, this results in a significant increase in atomic radius with the first elements of each period.
The atomic radius of each element generally decreases across each period due to an increasing number of protons, since an increase in the number of protons increases the attractive force acting on the atom's electrons. The greater attraction draws the electrons closer to the protons, decreasing the size of the atom. Down each group, the atomic radius of each element typically increases because there are more occupied
electron energy levels
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
and therefore a greater distance between protons and electrons.
The increasing nuclear charge is partly counterbalanced by the increasing number of electrons—a phenomenon that is known as shielding—which explains why the size of atoms usually increases down each column despite an increase in attractive force from the nucleus. Electron shielding causes the attraction of an atom's nucleus on its electrons to decrease, so electrons occupying higher energy states farther from the nucleus experience reduced attractive force, increasing the size of the atom. However, elements in the 5d-block (lutetium
Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
to mercury) are much smaller than this trend predicts due to the weak shielding of the 4f-subshell. This phenomenon is known as the lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii and ionic radii of the elements in the lanthanide series, from left to right. It is caused by the poor shielding effect of nuclear charge by the 4f electrons alo ...
. A similar phenomenon exists for actinides
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
; however, the general instability of transuranic element
The transuranium (or transuranic) elements are the chemical elements with atomic number greater than 92, which is the atomic number of uranium. All of them are radioactively unstable and decay into other elements. Except for neptunium and pluton ...
s makes measurements for the remainder of the 5f-block difficult and for transactinides nearly impossible. Finally, for sufficiently heavy elements, the atomic radius may be decreased by relativistic effects. This is a consequence of electrons near the strongly charged nucleus traveling at a sufficient fraction of the speed of light to gain a nontrivial amount of mass.
The following table summarizes the main phenomena that influence the atomic radius of an element:
Lanthanide contraction
The electrons in the 4f- subshell, which is progressively filled fromlanthanum
Lanthanum is a chemical element; it has symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements bet ...
('' Z'' = 57) to ytterbium
Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthani ...
(''Z'' = 70), are not particularly effective at shielding the increasing nuclear charge from the sub-shells further out. The elements immediately following the lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (el ...
s have atomic radii which are smaller than would be expected and which are almost identical to the atomic radii of the elements immediately above them.
Hence lutetium
Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
is in fact slightly smaller than yttrium
Yttrium is a chemical element; it has Symbol (chemistry), symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost a ...
, hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
has virtually the same atomic radius (and chemistry) as zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
, and tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition ...
has an atomic radius similar to niobium
Niobium is a chemical element; it has chemical symbol, symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and Ductility, ductile transition metal. Pure niobium has a Mohs scale of mineral hardness, Mohs h ...
, and so forth. The effect of the lanthanide contraction is noticeable up to platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
(''Z'' = 78), after which it is masked by a relativistic effect
Relativistic quantum chemistry combines relativistic mechanics with quantum chemistry to calculate elemental properties and structure, especially for the heavier elements of the periodic table. A prominent example is an explanation for the color of ...
known as the inert-pair effect.
Due to lanthanide contraction, the 5 following observations can be drawn:
# The size of Ln3+ ions regularly decreases with atomic number. According to Fajans' rules
In inorganic chemistry, Fajans' rules, formulated by Kazimierz Fajans in 1923, are used to predict whether a chemical bond will be covalent bond, covalent or ionic bond, ionic, and depend on the charge on the cation and the relative sizes of the ca ...
, decrease in size of Ln3+ ions increases the covalent character and decreases the basic character between Ln3+ and OH− ions in Ln(OH)3, to the point that Yb(OH)3 and Lu(OH)3 can dissolve with difficulty in hot concentrated NaOH. Hence the order of size of Ln3+ is given: La3+ > Ce3+ > ..., ... > Lu3+. # There is a regular decrease in their ionic radii. # There is a regular decrease in their tendency to act as a reducing agent, with an increase in atomic number. # The second and third rows of d-block transition elements are quite close in properties. # Consequently, these elements occur together in natural minerals and are difficult to separate.
d-block contraction
The d-block contraction is less pronounced than the lanthanide contraction but arises from a similar cause. In this case, it is the poor shielding capacity of the 3d-electrons which affects the atomic radii and chemistries of the elements immediately following the first row of thetransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s, from gallium
Gallium is a chemical element; it has Chemical symbol, symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875,
elemental gallium is a soft, silvery metal at standard temperature and pressure. ...
(''Z'' = 31) to bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
(''Z'' = 35).
Calculated atomic radius
The following table shows atomic radii computed from theoretical models, as published byEnrico Clementi
Enrico Clementi (November 19, 1931 in Cembra, Italy - March 30, 2021) was an Italian chemist, a pioneer in computational techniques for quantum chemistry and molecular dynamics.
Dr. Clementi received his Ph.D. in Chemistry from University of Pav ...
and others in 1967.
The values are in picometres (pm).
See also
*Atomic radii of the elements (data page)
The atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius ...
*Chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
*Covalent radius
The covalent radius, ''r''cov, is a measure of the size of an atom that forms part of one covalent bond. It is usually measured either in picometres (pm) or angstroms (Å), with 1 Å = 100 pm.
In principle, the sum of the two cova ...
*Bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
*Steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivi ...
*Kinetic diameter
Kinetic diameter is a measure applied to atoms and molecules that expresses the likelihood that a molecule in a gas will collide with another molecule. It is an indication of the size of the molecule as a target. The kinetic diameter is not the s ...
References
{{DEFAULTSORT:Atomic Radius Atomic radius Properties of chemical elements