Alpha-silicon Effect on:
[Wikipedia]
[Google]
[Amazon]
Generally speaking, second-row elements such as  Moreover, there is also another kind of silicon-α-effect, which is mainly about the
Moreover, there is also another kind of silicon-α-effect, which is mainly about the
 It is known that the
It is known that the
 After considering those properties above, researchers conclude the reasons for the first-kind Silicon-α-Effect. 3rd period heteroatoms can stabilize adjacent carbanions charges via hyperconjugation (this is sometimes called ''
After considering those properties above, researchers conclude the reasons for the first-kind Silicon-α-Effect. 3rd period heteroatoms can stabilize adjacent carbanions charges via hyperconjugation (this is sometimes called ''

silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
(Si) are known to stabilize α-carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3CH ...
s with greater effectiveness than a first-row element, which also means Si could destablize the α-carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encoun ...
s. This effect is known as silicon alpha effect. Another term that always associates with silicon alpha effect is the so-called silicon beta effect, which means Si at the β position could support formation of carbocations.
 Moreover, there is also another kind of silicon-α-effect, which is mainly about the
Moreover, there is also another kind of silicon-α-effect, which is mainly about the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
on the silicon atom. This effect could greatly enhance the hydrolysis rate on silicon and have a lot of practical applications in sealant
Sealant is a substance used to block the passage of fluids through openings in materials, a type of mechanical seal. In building construction ''sealant'' is sometimes synonymous with ''caulking'' and also serve the purposes of blocking dust, sou ...
s, adhesives, coating
A coating is a covering that is applied to the surface of an object, usually referred to as the substrate. The purpose of applying the coating may be decorative, functional, or both. Coatings may be applied as liquids, gases or solids e.g. Powde ...
s, and spray foam
Spray foam is a chemical product created by two materials, isocyanate and polyol resin, which react when mixed with each other and expand up to 30-60 times its liquid volume after it is sprayed in place. This expansion makes it useful as a specia ...
s.
History
In 1946, Sommer and co-workers reported that under conditions of basic hydrolysis, the alpha C-Cl bonds in trichloro(1-chloroethyl)silane (Cl3SiCHClCH3) and trichloro(1-chloropropyl)silane (Cl3SiCHClCH2CH3) failed to hydrolyze, while the beta C-Cl bonds in the analogous 2-chloroethyl and 2-chloropropyl compounds (Cl3SiCH2CH2Cl, Cl3SiCH2CHClCH3) reacted rapidly under the same conditions. They then concluded that silicon inhibits electrofugal activity at the alpha carbon. However, this conclusion seems to be contrary to the common sense in chemistry since theelectronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
of Si is lower than carbon (C), indicating the electron donating properties of Si. As a result of such confusion, many scientists are devoted to finding the answer to such phenomenon. In 1994, Yong and coworkers conducted first experiment to enable comparisons of the effects of α- and β-Si(CH3)3 moieties on the free energies of C-H homolysis and C-H heterolysis using thermodynamic data. They gave a solid conclusion that the β-silicon atom could help stabilize the carbocations and α-silicon atom will destablize the carbocations. However, the mechanisms under this phenomena still need more discussion.
Properties of bonds to silicon
In order to get a better understanding of the role of silicon in stabilizing the α-carbanions or destabilizing the α-carbocations, some general properties of silicon against carbon should be rationalized first.Electronegativity
 It is known that the
It is known that the electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
of Si (1.90) is lower than C (2.55) and the Si-C bond will be polarized, making Si susceptible to nucleophilic attack.
Bond length
The Si–C bond (1.89 Å) is significantly longer than a typical C–C bond (1.54 Å), suggesting that the SiMe3 group is sterically less demanding than atert-butyl group
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, givi ...
.
Bond energy
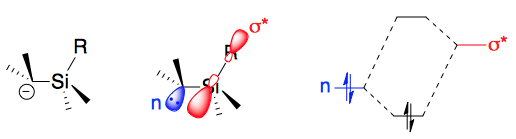
Single bonds from Si to electronegative elements O, Cl, and F are very strong. Since such strong bonds are formed, substitution at Si is especially easy when the nucleophile is O-, Cl-, or F-.Negative hyperconjugation
negative hyperconjugation
In organic chemistry, negative hyperconjugation is the donation of electron density from a filled π- or p-orbital to a neighboring σ*-orbital. This phenomenon, a type of resonance, can stabilize the molecule or transition state. It also cause ...
''). For the case in silicon, it could be depicted as the picture oon the right. The σ* orbitals of Si could help to stablize the electrons in carbon, enhancing the formation of carbanions.
Hydrolysis on silicon
The silicon α‑effect described above is mainly focused on carbon. In fact, there is another kind so-called silicon α‑effect, which is focused on silicon. It describes another phenomenon that under hydrolysis condition, certain α-silane-terminated prepolymers crosslink 10-1000 times faster than the corresponding prepolymers produced from conventional Cγ-functionalized trialkoxypropylsilanes and dialkoxymethylpropylsilanes.History
This silicon α-effect was first observed in the late 1960s by researchers atBayer AG
Bayer AG (, commonly pronounced ; ) is a German multinational pharmaceutical and biotechnology company and one of the largest pharmaceutical companies in the world. Headquartered in Leverkusen, Bayer's areas of business include pharmaceutica ...
as an increase in reactivity at the silicon atom for hydrolysis and was used for cross-linking of α-silane-terminated prepolymers. For a long time after that, people attributed this reactivity as silicon α-effect. However, the real mechanism beneath it had been debated for many years after this discovery. Generally, this effect has been rationalized as an intramolecular donor- acceptor interaction between the lone pair of the organofunctional group (such as NR2, OC(O)R, N(H)COOMe) and the silicon atom. However, this hypothesis has been proved incorrect by Mitzel and coworkers and more experiments are needed to interpret this effect.
Mechanism study
Reinhold and coworkers performed a systematical experiment to study the kinetics and mechanisms of hydrolysis of such compounds. They prepared a series of α-silanes and γ-silanes and tested their reactivity in different pH (acidic and basic regime), functional group X and the spacer between the silicon atom and the functional group X. In general, they find that under basic conditions, the rate of hydrolysis is mainly controlled by the electrophilicity of the silicon center and the rate of the hydrolysis of the γ-silanes is less influenced by the generally electronegative functional groups than α-silanes. More electronegative the functional groups are, the higher the rate of hydrolysis. However, under acidic conditions, the rate of hydrolysis depends on both the electrophilicity of the silicon center (determining the molecular reactivity) and the concentration of the (protonated) reactive species. Under acidic conditions, the nucleophile changes from OH- to H2O, so it involves the process of protonation and the atoms are protonated could be either silicon or the functional group X. As a result, the general trend in acidic solution is more complicated.References
{{Reflist Silicon