|
Verruculogen
Verruculogen is a mycotoxin produced by certain strains of ''aspergillus'' that belongs to a class of naturally occurring 2,5-diketopiperazines. It is an annulated analogue of cyclo(L-Trp-L-Pro) which belongs to the most abundant and structurally diverse class of tryptophan-proline 2,5-diketopiperazine natural products. It produces tremors in mice due to its neurotoxic properties. It also tested positive in a ''Salmonella''/mammalian microsome assay and was shown to be genotoxic. It is a potent blocker of calcium-activated potassium channels.http://www.sigmaaldrich.com/catalog/product/sigma/v7755?lang=en®ion=US Synthesis Both verruculogen and its isoprenyl derivative fumitremorgin A belong to the only family of alkaloids with an eight-membered endoperoxide ring, and both have been synthesised involving ligand-controlled C–H borylation Metal-catalyzed C–H borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycotoxin
A mycotoxin (from the Greek μύκης , "fungus" and τοξίνη , "toxin") is a toxic secondary metabolite produced by organisms of kingdom Fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops. Examples of mycotoxins causing human and animal illness include aflatoxin, citrinin, fumonisins, ochratoxin A, patulin, trichothecenes, zearalenone, and ergot alkaloids such as ergotamine. One mold species may produce many different mycotoxins, and several species may produce the same mycotoxin. Production Most fungi are aerobic (use oxygen) and are found almost everywhere in extremely small quantities due to the diminute size of their spores. They consume organic matter wherever humidity and temperature are sufficient. Where conditions are right, fungi proliferate into colonies and mycotoxin levels become high. The reason for the pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspergillus
'''' () is a genus consisting of several hundred mold species found in various climates worldwide. ''Aspergillus'' was first catalogued in 1729 by the Italian priest and biologist Pier Antonio Micheli. Viewing the fungi under a microscope, Micheli was reminded of the shape of an '' aspergillum'' (holy water sprinkler), from Latin ''spargere'' (to sprinkle), and named the genus accordingly. Aspergillum is an asexual spore-forming structure common to all ''Aspergillus'' species; around one-third of species are also known to have a sexual stage. While some species of ''Aspergillus'' are known to cause fungal infections, others are of commercial importance. Taxonomy Species ''Aspergillus'' consists of 837 species of fungi. Growth and distribution ''Aspergillus'' is defined as a group of conidial fungi—that is, fungi in an asexual state. Some of them, however, are known to have a teleomorph (sexual state) in the Ascomycota. With DNA evidence, all members of the genus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Annulated
In organic chemistry annulation (from the Latin ''anellus'' for "little ring"; occasionally annelation) is a chemical reaction in which a new ring is constructed on a molecule. : Examples are the Robinson annulation, Danheiser annulation and certain cycloadditions. Annular molecules are constructed from side-on condensed cyclic segments, for example helicenes and acenes. In transannulation a bicyclic molecule is created by intramolecular carbon-carbon bond formation in a large monocyclic ring. An example is the samarium(II) iodide induced ketone - alkene cyclization of ''5-methylenecyclooctanone'' which proceeds through a ketyl intermediate: : Benzannulation The term benzannulated compounds refers to derivatives of cyclic compounds (usually aromatic) which are fused to a benzene ring. Examples are listed in the table below: In contemporary chemical literature, the term benzannulation also means "construction of benzene rings from acyclic precursors". upright=1.4, Protonatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Genotoxic
Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, but some genotoxic substances are not mutagenic. The alteration can have direct or indirect effects on the DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. The permanent, heritable changes can affect either somatic cells of the organism or germ cells to be passed on to future generations. Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis. To assay for genotoxic molecules, researchers assay for DNA damage in cells exposed to the toxic substrates. This DNA damage can be in the form of single- and double-strand breaks, loss of excision repair, cross-linking, alkali-labile sites, point mutations, and st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
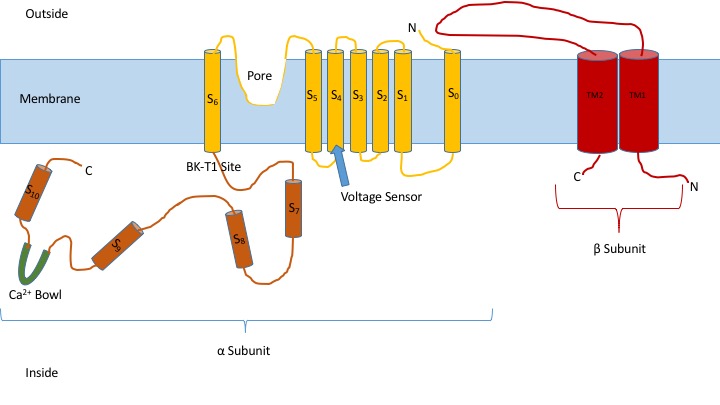
Calcium-activated Potassium Channel
Calcium-activated potassium channels are potassium channels gated by calcium, or that are structurally or phylogenetically related to calcium gated channels. They were first discovered in 1958 by Gardos who saw that calcium levels inside of a cell could affect the permeability of potassium through that cell membrane. Then in 1970, Meech was the first to observe that intracellular calcium could trigger potassium currents. In humans they are divided into three subtypes: large conductance or BK channels, which have very high conductance which range from 100 to 300 pS, intermediate conductance or IK channels, with intermediate conductance ranging from 25 to 100 pS, and small conductance or SK channels with small conductances from 2-25 pS. This family of ion channels is, for the most part, activated by intracellular Ca2+ and contains 8 members in the human genome. However, some of these channels (the KCa4 and KCa5 channels) are responsive instead to other intracellular ligands, such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fumitremorgin
Fumitremorgin A-C Fumitremorgins are tremorogenic metabolites of ''Aspergillus'' and ''Penicillium'', that belong to a class of naturally occurring 2,5-diketopiperazines. Biosynthesis Biosynthesis pathway of fumitremorgin pathway involves several different enzymes. FtmA is a nonribosomal peptide synthase. Both FtmB and FtmH are prenyltransferase. Three different cytochrome P450 monooxygenases involved in the biosynthesis of furmitremorgin C are FtmC, FtmE, and FtmG. Furthermore, FtmD is proposed to function as the methyltransferase. The synthesis starts with the formation of brevianamide F. FtmA catalyzes the nonribosomal peptide synthesis (NRPS) of this diketopiperazine product from two amino acids, L-tryptophan and L-proline. Then, another enzyme, FtmB, prenylates the product to form tryprostatin B. At this point, there are two separate pathways. FtmE may cyclize tryprostatin B to form demethoxyfumitremorgin C, or FtmC may oxidize tryprostatin B to form desmethyltrprostatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borylation
Metal-catalyzed C–H borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through functionalization of aliphatic and aromatic C–H bonds and are therefore useful reactions for carbon–hydrogen bond activation. Metal-catalyzed C–H borylation reactions utilize transition metals to directly convert a C–H bond into a C–B bond. This route can be advantageous compared to traditional borylation reactions by making use of cheap and abundant hydrocarbon starting material, limiting prefunctionalized organic compounds, reducing toxic byproducts, and streamlining the synthesis of biologically important molecules. Boronic acids, and boronic esters are common boryl groups incorporated into organic molecules through borylation reactions. Boronic acids are trivalent boron-containing organic compounds that possess one alkyl substituent and two hydroxyl groups. Similarly, boronic esters possess one alkyl substituent and two ester groups. B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycotoxins
A mycotoxin (from the Greek μύκης , "fungus" and τοξίνη , "toxin") is a toxic secondary metabolite produced by organisms of kingdom Fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops. Examples of mycotoxins causing human and animal illness include aflatoxin, citrinin, fumonisins, ochratoxin A, patulin, trichothecenes, zearalenone, and ergot alkaloids such as ergotamine. One mold species may produce many different mycotoxins, and several species may produce the same mycotoxin. Production Most fungi are aerobic (use oxygen) and are found almost everywhere in extremely small quantities due to the diminute size of their spores. They consume organic matter wherever humidity and temperature are sufficient. Where conditions are right, fungi proliferate into colonies and mycotoxin levels become high. The reason for the producti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diketopiperazines
A diketopiperazine (DKP), also known as a ''dioxopiperazine'' or ''piperazinedione'', is a class of organic compounds related to piperazine but containing two amide linkages. DKP's are the smallest known class of cyclic peptide. Despite their name, they are not ketones, but amides. Three regioisomers are possible, differing in the locations of the carbonyl groups. * One isomer is an oxamide obtained from ethylenediamine. * 2,5-Diketopiperazines are cyclodipeptides often obtainable via condensation of two α-amino acids. * 2,6-Diketopiperazines may be viewed as cyclized imide derivatives derived from iminodiacetic acids. Of these three isomeric diketopiperazines, the 2,5-derivatives have attracted the greatest interest. Due to their appearance in biologically active natural products, medicinal chemists have been inspired to use DKPs to circumvent the poor physical and metabolic properties of peptides in the course of drug discovery. Natural sources DKPs are synthesized by a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Peroxides
In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily breaks, producing free radicals of the form (the dot represents an unpaired electron). Thus, organic peroxides are useful as initiators for some types of polymerisation, such as the epoxy resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can explosively combust. Organic peroxides, like their inorganic counterparts, are often powerful bleaching agents. Types of organic peroxides Tert-Butyl hydroperoxide Structural Formula V2.svg, ''tert''-Butyl hydroperoxide, a hydroperoxide (formula: ROOH) that is used to epoxide alkenes. Dicumyl peroxide.svg, Dicumyl peroxide, a dialkyl peroxide (formula: ROOR) that is used to initiate po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |