|
Vicryl
Vicryl (polyglactin 910) is an absorbable, synthetic, usually braided surgical suture, suture, manufactured by Ethicon Inc., a subsidiary of Johnson and Johnson. A monofilament version is also made for use in Ophthalmology, ophthalmic practice. It is indicated for soft tissue approximation and Ligature (medicine), ligation. The suture holds its tensile strength for approximately two to three weeks in tissue and is completely absorbed by acid hydrolysis within 8-10 weeks. Vicryl and other Polyglycolide, polyglycolic-acid sutures may also be treated for more rapid breakdown ("Vicryl Rapide") in rapidly healing tissues such as mucous membrane or impregnated with triclosan ("Vicryl Plus Antibacterial") to provide antimicrobial protection of the suture line. Because Vicryl is slow-absorbing and often braided, its use is contraindicated in the closure of any cutaneous wound exposed to the air, as it draws moisture from the healing tissue to the skin and allows bacteria and irritants t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vicryl Rapide
Vicryl (polyglactin 910) is an absorbable, synthetic, usually braided suture, manufactured by Ethicon Inc., a subsidiary of Johnson and Johnson. A monofilament version is also made for use in ophthalmic practice. It is indicated for soft tissue approximation and ligation. The suture holds its tensile strength for approximately two to three weeks in tissue and is completely absorbed by acid hydrolysis within 8-10 weeks. Vicryl and other polyglycolic-acid sutures may also be treated for more rapid breakdown (" Vicryl Rapide") in rapidly healing tissues such as mucous membrane or impregnated with triclosan (" Vicryl Plus Antibacterial") to provide antimicrobial protection of the suture line. Because Vicryl is slow-absorbing and often braided, its use is contraindicated in the closure of any cutaneous wound exposed to the air, as it draws moisture from the healing tissue to the skin and allows bacteria and irritants to migrate into the wound. This may lead to high reactivity to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
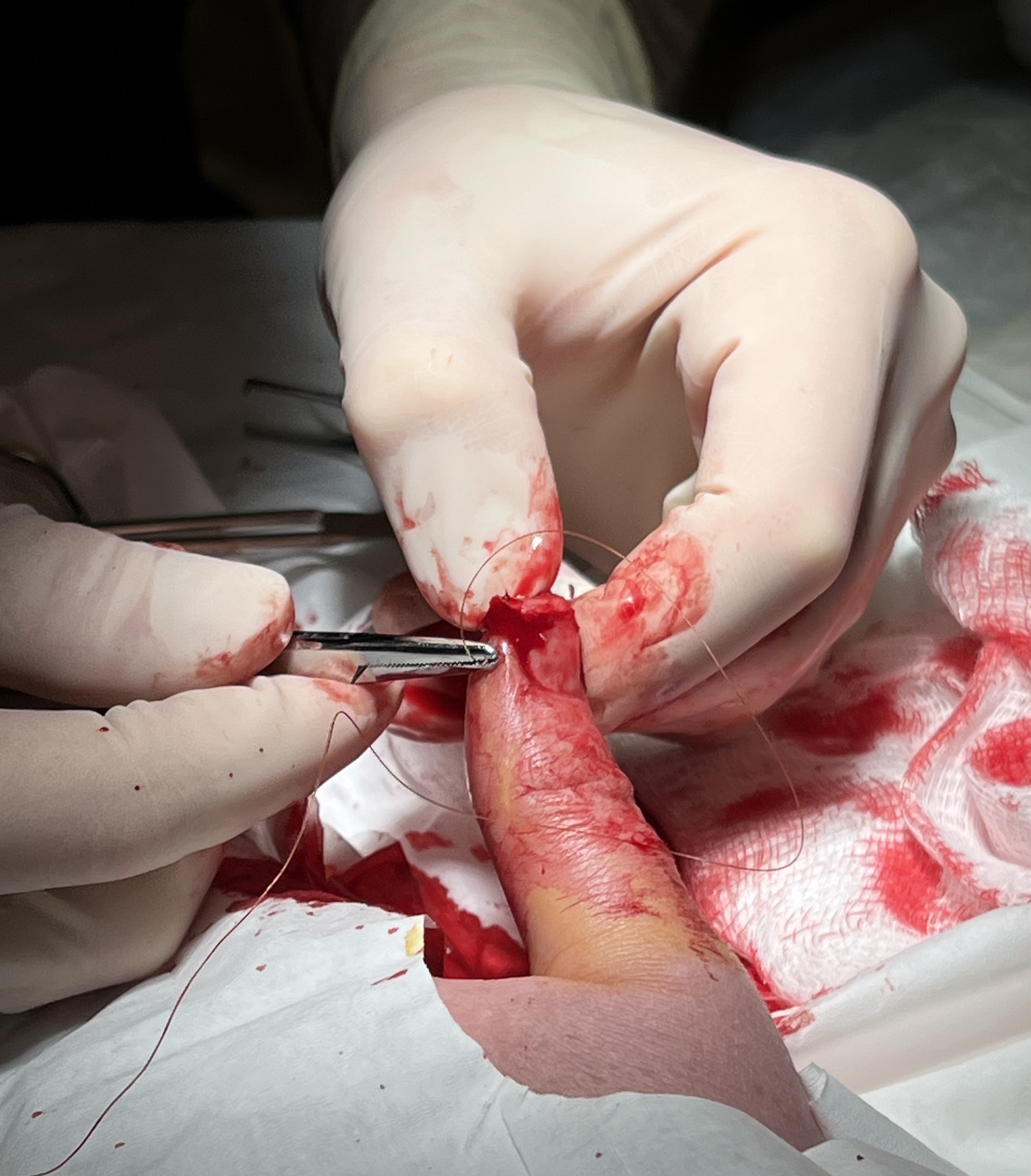
Surgical Suture
A surgical suture, also known as a stitch or stitches, is a medical device used to hold Tissue (biology), body tissues together and approximate wound edges after an injury or surgery. Application generally involves using a Sewing needle, needle with an attached length of thread (yarn), thread. There are numerous types of suture which differ by needle shape and size as well as thread material and characteristics. Selection of surgical suture should be determined by the characteristics and location of the wound or the specific body tissues being approximated. In selecting the needle, thread, and suturing technique to use for a specific patient, a medical care provider must consider the tensile strength of the specific suture thread needed to efficiently hold the tissues together depending on the mechanical and shear forces acting on the wound as well as the thickness of the tissue being approximated. One must also consider the elasticity of the thread and ability to adapt to differe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolide
Glycolide (1,4-dioxane-2,5-dione) is a dimer of glycolic acid. Its structure is six-membered ring containing two lactones, an oxidized variant of ''p''-dioxane. The compound can be synthesized from glycolic acid, via a high-temperature oligomerization to form polyglycolide followed by a depolymerization process. A more direct method is by self-condensation of the sodium salt of chloroacetic acid Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula . This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds are dichlo .... It is useful as a starting material for the production of polyglycolide. References Dimers (chemistry) Lactones Dioxanes {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactic Acid
Lactic acid is an organic acid. It has the molecular formula C3H6O3. It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate (or the lactate anion). The name of the derived acyl group is lactoyl. In solution, it can ionize by a loss of a proton to produce the lactate ion . Compared to acetic acid, its p''K'' is 1 unit less, meaning lactic acid is ten times more acidic than acetic acid. This higher acidity is the consequence of the intramolecular hydrogen bonding between the α-hydroxyl and the carboxylate group. Lactic acid is chiral, consisting of two en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactone
Lactones are cyclic carboxylic esters. They are derived from the corresponding hydroxycarboxylic acids by esterification. They can be saturated or unsaturated. Lactones are formed by lactonization, the intramolecular esterification of the corresponding hydroxycarboxylic acids. Nomenclature Greek alphabet#Letters, Greek prefixes in alphabetical order indicate ring size. Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between the relevant -OH and the -COOH groups along said backbone. The first carbon atom after the carbon in the -COOH group on the parent compound is labelled α, the second will be labeled β, and so forth. Therefore, the prefixes also indicate the size of the lactone ring: α-lactone = 3-membered ring, β-lac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactide
Lactide is the lactone cyclic ester derived by multiple esterification between two (usually) or more molecules from lactic acid (2-hydroxypropionic acid) or other hydroxy carboxylic acid. They are designated as dilactides, trilactides, etc., according to the number of hydroxy acid residues. All lactides are colorless or white solids. The dilactide derived from lactic acid has the formula . This lactide has attracted interest because it is derived from abundant renewable resources and is the precursor to a biodegradable plastic. Stereoisomers The dilactide derived from lactic acid can exist in three different stereoisomeric forms. This complexity arises because lactic acid is chiral. These enantiomers do not racemize readily. All three stereoisomers undergo epimerisation in the presence of organic and inorganic bases in solution. Polymerization Lactide can be polymerized to polylactic acid (polylactide). Depending on the catalyst Catalysis () is the increase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dexon
Polyglycolide or poly(glycolic acid) (PGA), also spelled as polyglycolic acid, is a biodegradable, thermoplastic polymer and the simplest linear, aliphatic polyester. It can be prepared starting from glycolic acid by means of polycondensation or ring-opening polymerization. PGA has been known since 1954 as a tough fiber-forming polymer. Owing to its hydrolytic instability, however, its use was slow to develop. Polyglycolide and its copolymers ( poly(lactic-''co''-glycolic acid) with lactic acid, poly(glycolide-''co''-caprolactone) with ε-caprolactone and poly (glycolide-''co''-trimethylene carbonate) with trimethylene carbonate) are widely used as a material for the synthesis of absorbable sutures and are being evaluated in the biomedical field. Physical properties Polyglycolide has a glass transition temperature between 35 and 40 °C and a melting point in the range of 225 to 230 °C. PGA also exhibits an elevated degree of crystallinity, around 45–55%, thus res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |