|
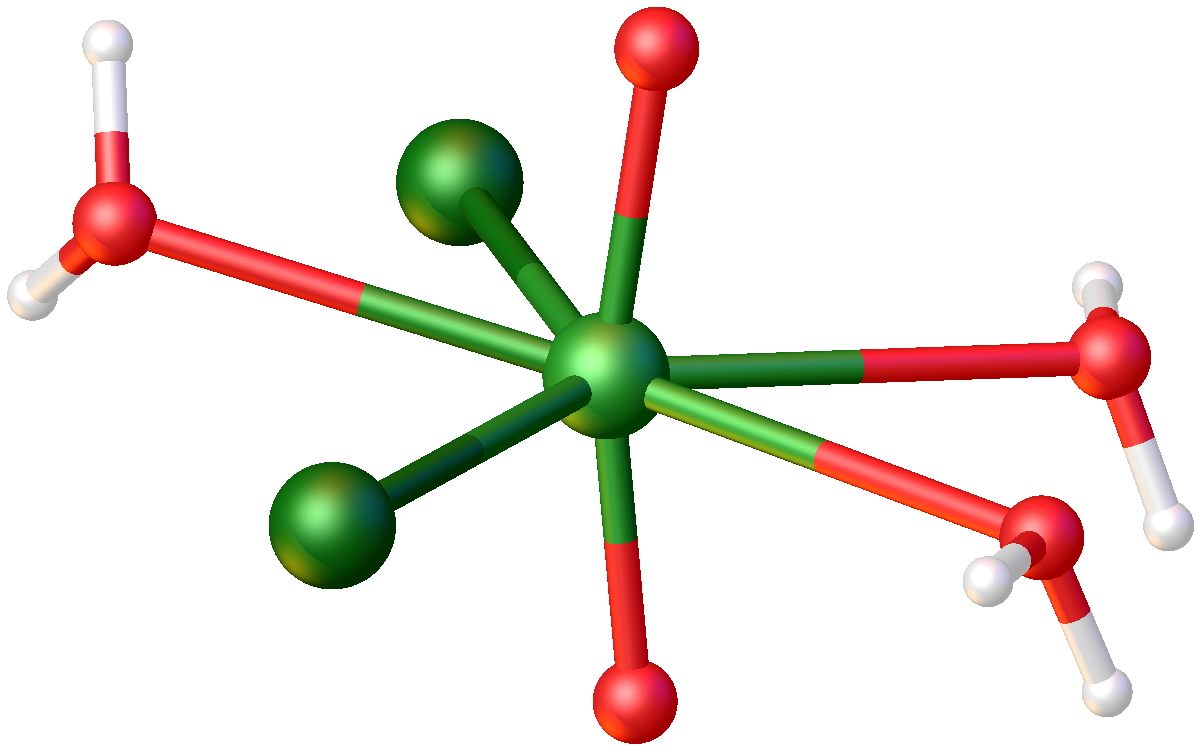
Uranyl Fluoride
Uranyl fluoride is the inorganic compound with the formula . It is most notable as a contaminant in the production of uranium tetrafluoride. As shown by X-ray crystallography, the uranyl centers are surrounded by six fluoride ligands . This salt is very soluble in water as well as hygroscopic. It changes in color from brilliant orange to yellow after reacting with water. Uranyl fluoride is stable in air up to 300 °C, above which slow decomposition to occurs. When heated to decomposition, emits toxic hydrofluoric acid fumes. It is formed in the hydrolysis of uranium hexafluoride (): : It can also be formed in the hydrofluorination of uranium trioxide (): : References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Uranyl Chloride
Uranyl chloride is a chemical compound with the chemical formula . It consists of uranyl cations and chloride anions . It is fluorescent. Uranyl chloride also refers to inorganic compounds with the formula where ''n'' = 0, 1, 2, or 3. These are yellow salts. Synthesis and structures The hydrates are obtained by dissolving uranyl sulfate or uranyl acetate in hydrochloric acid followed by crystallization from concentrated solutions. Depending on the method of drying, one obtains the mono- or the trihydrate. The monohydrate is described as a yellow, sulfur-like powder. It is very hygroscopic. The trihydrate is greenish-yellow. Both hydrates are fluorescent solids that are highly soluble in water. The anhydrous material can be obtained by the reaction of oxygen with uranium tetrachloride: : In terms of structures, all three of these compounds feature the uranyl center (''trans''-) bound to five additional ligands, which can include (bridging) chloride, water, or another uranyl oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Inorganic Compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep Mantle (geology), mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salt (chemistry), salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within life, living things. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Uranium Tetrafluoride
Uranium tetrafluoride is the inorganic compound with the formula UF4. It is a green solid with an insignificant vapor pressure and low solubility in water. Uranium in its tetravalent ( uranous) state is important in various technological processes. In the uranium refining industry it is known as green salt. Production UF4 is prepared from UO2 in a fluidized bed by reaction with Hydrogen fluoride. The UO2 is derived from mining operations. Around 60,000 tonnes are prepared in this way annually. A common impurity is UO2F2. UF4 is susceptible to hydrolysis as well. UF4 is formed by the reaction of UF6 with hydrogen gas in a vertical tube-type reactor. The bulk density of UF4 varies from about 2.0 g/cm3 to about 4.5 g/cm3 depending on the production process and the properties of the starting uranium compounds. A molten salt reactor design, a type of nuclear reactor where the working fluid is a molten salt, would use UF4 as the core material. UF4 is generally chosen over relate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
X-ray Crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallography, crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information. X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences between various materials, especially minerals and alloys. The method has also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption (chemistry), absorption or adsorption from the surrounding Natural environment, environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g. changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they dissolve in the water they absorb, forming an aqueous solution. Hygroscopy is essential for many plant and animal species' attainment of hydration, nutrition, reproduction and/or seed dispersal. Biological evolution created hygroscopic solutions for water harvesting, filament tensile strength, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
U3O8
Triuranium octoxide (U3O8) is a compound of uranium. It is present as an olive green to black, odorless solid. It is one of the more popular forms of yellowcake and is shipped between mills and refineries in this form. U3O8 has potential long-term stability in a geologic environment. In the presence of oxygen (O2), uranium dioxide (UO2) is oxidized to U3O8, whereas uranium trioxide (UO3) loses oxygen at temperatures above 500 °C and is reduced to U3O8. The compound can be produced by the calcination of ammonium diuranate or ammonium uranyl carbonate. Due to its high stability, it can be used for the disposal of depleted uranium.United States Nuclear Regulatory Commission, "Frequently Asked Questions about Depleted Uranium Deconversion Facilities", https://www.nrc.gov/materials/fuel-cycle-fac/ur-deconversion/faq-depleted-ur-decon.html Its particle density is 8.38 g cm−3. Triuranium octoxide is converted to uranium hexafluoride for the purpose of uranium enrichment. Format ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydrofluoric Acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling point near room temperature. It is used to make most organofluorine compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Uranium Hexafluoride
Uranium hexafluoride, sometimes called hex, is the inorganic compound with the formula . Uranium hexafluoride is a volatile, white solid that is used in enriching uranium for nuclear reactors and nuclear weapons. Preparation Uranium dioxide is converted with hydrofluoric acid (HF) to uranium tetrafluoride: : The resulting is subsequently oxidized with fluorine to give the hexafluoride: : In samples contaminated with uranium trioxide, uranyl fluoride, an oxyfluoride compound is produced in the HF step: : which can be fluorinated to produce the same product, uranium hexafluoride. : The fluorination step in both reactions above are highly exothermic. Properties Physical properties At atmospheric pressure, sublimes at 56.5 °C. The solid-state structure was determined by neutron diffraction at 77 K and 293 K.J. C. Taylor, P. W. Wilson, J. W. Kelly: „The structures of fluorides. I. Deviations from ideal symmetry in the structure of crystalline UF6: a neutron diffract ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Uranium Trioxide
Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph is amorphous UO3. Production and use There are three methods to generate uranium trioxide. As noted below, two are used industrially in the reprocessing of nuclear fuel and uranium enrichment. # U3O8 can be oxidized at 500 °C with oxygen. Note that above 750 °C even in 5 atm O2 UO3 decomposes into U3O8. # Uranyl nitrate, UO2(NO3)2·6H2O can be heated to yield UO3. This occurs during the reprocessing of nuclear fuel. Fuel rods are dissolved in HNO3 to separate uranyl nitrate from plutonium and the fission products (the PUREX method). The pure uranyl nitrate is converted to solid UO3 by heating at 400 °C. After reduction with hydrogen (with other inert gas present) to uranium dioxide, the uranium can be used in new MOX fuel rod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Uranyl Compounds
The uranyl ion with the chemical formula has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen, with uranium in the oxidation state +6. Four or more ligands may be bound to the uranyl ion in an equatorial plane around the uranium atom. The uranyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing. Structure and bonding The uranyl ion is linear and symmetrical, specifically belonging to the D∞h point group, with both U–O bond lengths of about 180 pm. The bond lengths are indicative of the presence of multiple bonding between the uranium and oxygen atoms. Since uranium(VI) has the electronic configuration of the preceding noble gas, radon, the electrons used in forming the U–O bonds are supplied by the oxygen atoms. The electrons are donated into empty atomic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Metal Halides
Metal halides are compounds between metals and halogens. Some, such as sodium chloride are Ionic compound, ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride. File:NaCl polyhedra.svg, Sodium chloride crystal structure File:Uranium-hexafluoride-unit-cell-3D-balls.png, Discrete UF6 molecules File:Alpha-palladium(II)-chloride-xtal-3D-balls.png, Infinite chains of one form of palladium chloride Preparation The halogens can all react with metals to form metal halides according to the following equation: :2M + nX2 → 2MXn where M is the metal, X is the halogen, and MXn is the metal halide. In practice, this type of reaction may be very exothermic, hence impractical as a preparative technique. Additionally, many transition metals can adopt multiple oxidation states, which complicates matters. As the halogens are strong oxidizers, direct combination of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |