|
Unsaturated
Saturation, saturated, unsaturation or unsaturated may refer to: Chemistry * Saturation, a property of organic compounds referring to carbon-carbon bonds **Saturated and unsaturated compounds **Degree of unsaturation **Saturated fat or fatty acid **Unsaturated fat or fatty acid * Non-susceptibility of an organometallic compound to oxidative addition * Saturation of protein binding sites * Saturation of enzymes with a substrate * Saturation of a solute in a solution, as related to the solute's maximum solubility at equilibrium ** Supersaturation, where the concentration of a solute exceeds its maximum solubility at equilibrium ** Undersaturation, where the concentration of a solute is less than its maximum solubility at equilibrium Biology * Oxygen saturation, a clinical measure of the amount of oxygen in a patient's blood * Saturation pollination, a pollination technique * Saturated mutagenesis, a form of site-directed mutagenesis * Saturation (genetic), the observed number of m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unsaturated Fat
An unsaturated fat is a fat or fatty acid in which there is at least one double bond within the fatty acid chain. A fatty acid chain is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond. Where double bonds are formed, hydrogen atoms are subtracted from the carbon chain. Thus, a saturated fat has no double bonds, has the maximum number of hydrogens bonded to the carbons, and therefore is "saturated" with hydrogen atoms. In cellular metabolism, unsaturated fat molecules contain somewhat less energy (i.e., fewer calories) than an equivalent amount of saturated fat. The greater the degree of unsaturation in a fatty acid (i.e., the more double bonds in the fatty acid) the more vulnerable it is to lipid peroxidation ( rancidity). Antioxidants can protect unsaturated fat from lipid peroxidation. Chemistry and nutrition Double bonds may be in either a ''cis'' or a ''trans'' isomer, depending on the geometry of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated Fat
A saturated fat is a type of fat in which the fatty acid chains have all single bonds. A fat known as a glyceride is made of two kinds of smaller molecules: a short glycerol backbone and fatty acids that each contain a long linear or branched chain of carbon (C) atoms. Along the chain, some carbon atoms are linked by single bonds (-C-C-) and others are linked by double bonds (-C=C-). A double bond along the carbon chain can react with a pair of hydrogen atoms to change into a single -C-C- bond, with each H atom now bonded to one of the two C atoms. Glyceride fats without any carbon chain double bonds are called saturated because they are "saturated with" hydrogen atoms, having no double bonds available to react with more hydrogen. Most animal fats are saturated. The fats of plants and fish are generally unsaturated. Various foods contain different proportions of saturated and unsaturated fat. Many processed foods like foods deep-fried in hydrogenated oil and sausage are hig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated And Unsaturated Compounds
In chemistry, a saturated compound is a chemical compound (or ion) that resists the addition reactions, such as hydrogenation, oxidative addition, and binding of a Lewis base. The term is used in many contexts and for many classes of chemical compounds. Overall, saturated compounds are less reactive than unsaturated compounds. Saturation is derived from the Latin word ''saturare'', meaning 'to fill'. Organic chemistry Unsaturated compounds generally carry out typical addition reactions that are not possible with saturated compounds such as alkanes. A saturated organic compound has only single bonds between carbon atoms. An important class of saturated compounds are the alkanes. Many saturated compounds have functional groups, e.g., alcohols. Unsaturated organic compounds The concept of saturation can be described using various naming systems, formulas, and analytical tests. For instance, IUPAC nomenclature is a system of naming conventions used to describe the type an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Content
Water content or moisture content is the quantity of water contained in a material, such as soil (called soil moisture), rock, ceramics, crops, or wood. Water content is used in a wide range of scientific and technical areas, and is expressed as a ratio, which can range from 0 (completely dry) to the value of the materials' porosity at saturation. It can be given on a volumetric or mass (gravimetric) basis. Definitions Volumetric water content, θ, is defined mathematically as: :\theta = \frac where V_w is the volume of water and V_\text = V_s + V_w + V_a is equal to the total volume of the wet material, i.e. of the sum of the volume of solid host material (e.g., soil particles, vegetation tissue) V_s, of water V_w, and of air V_a. Gravimetric water content is expressed by mass (weight) as follows: :u = \frac where m_w is the mass of water and m_s is the mass of the solids. For materials that change in volume with water content, such as coal, the gravimetric water content, ''u' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
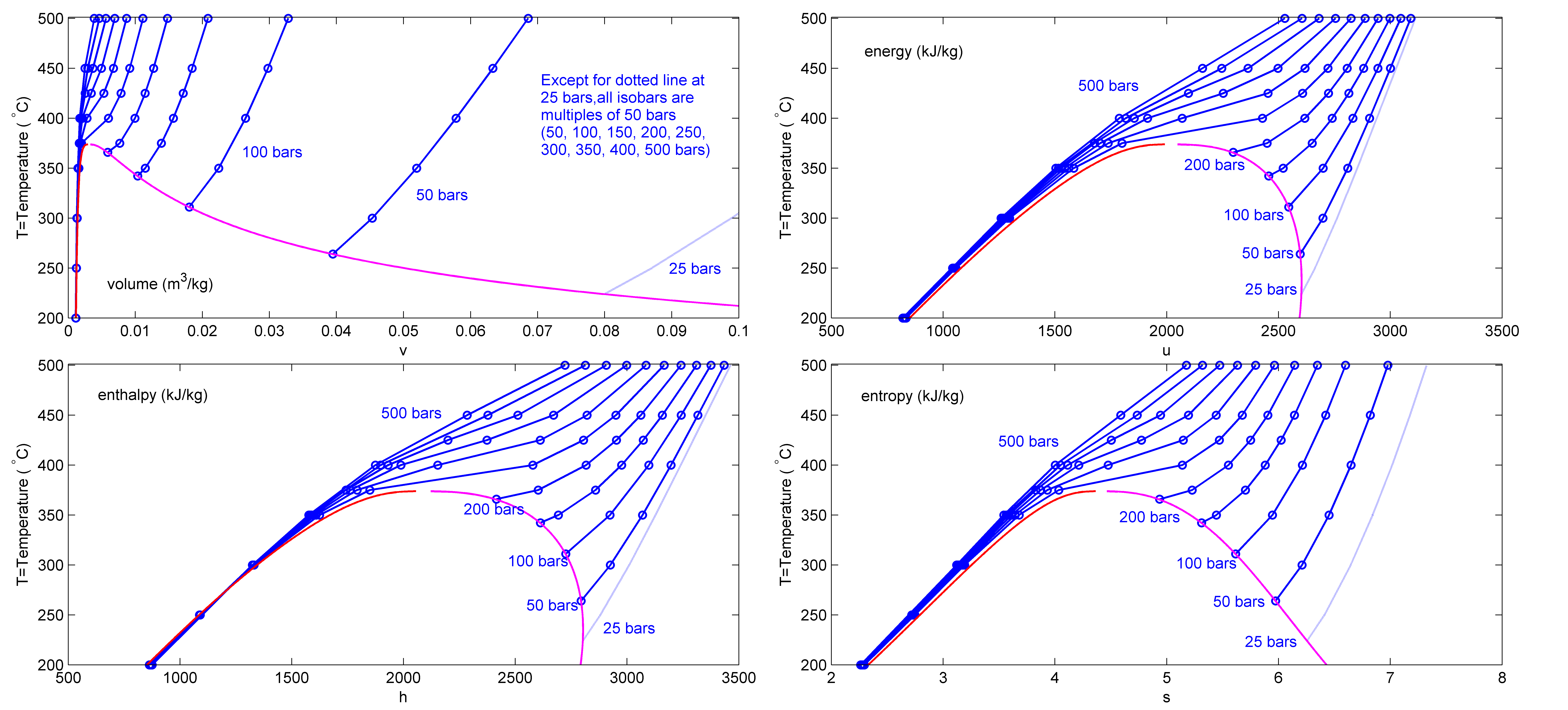
Saturated Fluid
Saturation, saturated, unsaturation or unsaturated may refer to: Chemistry * Saturation, a property of organic compounds referring to carbon-carbon bonds **Saturated and unsaturated compounds ** Degree of unsaturation **Saturated fat or fatty acid **Unsaturated fat or fatty acid * Non-susceptibility of an organometallic compound to oxidative addition * Saturation of protein binding sites * Saturation of enzymes with a substrate * Saturation of a solute in a solution, as related to the solute's maximum solubility at equilibrium ** Supersaturation, where the concentration of a solute exceeds its maximum solubility at equilibrium ** Undersaturation, where the concentration of a solute is less than its maximum solubility at equilibrium Biology * Oxygen saturation, a clinical measure of the amount of oxygen in a patient's blood * Saturation pollination, a pollination technique * Saturated mutagenesis, a form of site-directed mutagenesis * Saturation (genetic), the observed number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unsaturated Zone
The vadose zone, also termed the unsaturated zone, is the part of Earth between the land surface and the top of the phreatic zone, the position at which the groundwater (the water in the soil's pores) is at atmospheric pressure ("vadose" is from the Latin word for "shallow"). Hence, the vadose zone extends from the top of the ground surface to the water table. Water in the vadose zone has a pressure head less than atmospheric pressure, and is retained by a combination of adhesion (''funiculary groundwater''), and capillary action (''capillary groundwater''). If the vadose zone envelops soil, the water contained therein is termed soil moisture. In fine grained soils, capillary action can cause the pores of the soil to be fully saturated above the water table at a pressure less than atmospheric. The vadose zone does not include the area that is still saturated above the water table, often referred to as the capillary fringe. Freeze, R.A. and Cherry, J.A., 1979. Groundwater. Engle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturation (commutative Algebra)
Saturation, saturated, unsaturation or unsaturated may refer to: Chemistry * Saturation, a property of organic compounds referring to carbon-carbon bonds **Saturated and unsaturated compounds **Degree of unsaturation **Saturated fat or fatty acid **Unsaturated fat or fatty acid * Non-susceptibility of an organometallic compound to oxidative addition * Saturation of protein binding sites * Saturation of enzymes with a substrate * Saturation of a solute in a solution, as related to the solute's maximum solubility at equilibrium ** Supersaturation, where the concentration of a solute exceeds its maximum solubility at equilibrium ** Undersaturation, where the concentration of a solute is less than its maximum solubility at equilibrium Biology * Oxygen saturation, a clinical measure of the amount of oxygen in a patient's blood * Saturation pollination, a pollination technique * Saturated mutagenesis, a form of site-directed mutagenesis * Saturation (genetic), the observed number of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Degree Of Unsaturation
In the analysis of the molecular formula of organic molecules, the degree of unsaturation (also known as the index of hydrogen deficiency (IHD), double bond equivalents, or unsaturation index) is a calculation that determines the total number of rings and π bonds. A formula is used in organic chemistry to help draw chemical structures. It does not give any information about those components individually—the specific number of rings, or of double bonds (one π bond each), or of triple bonds (two π bonds each). The final structure is verified with use of NMR, mass spectrometry and IR spectroscopy, as well as qualitative inspection. It is based on comparing the actual molecular formula to what would be a possible formula if the structure were saturated—having no rings and containing only σ bonds—with all atoms having their standard valence. General formula The formula for degree of unsaturation is: :\mathrm = 1 + \tfrac \sum n_i(v_i-2) where ''ni'' is the number of atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superheated Steam
Superheated steam is steam at a temperature higher than its vaporization point at the absolute pressure where the temperature is measured. Superheated steam can therefore cool (lose internal energy) by some amount, resulting in a lowering of its temperature without changing state (i.e., condensing) from a gas, to a mixture of saturated vapor and liquid. If unsaturated steam (a mixture which contains both water vapor and liquid water droplets) is heated at constant pressure, its temperature will also remain constant as the vapor quality (think dryness, or percent saturated vapor) increases towards 100%, and becomes dry (i.e., no saturated liquid) saturated steam. Continued heat input will then "super" heat the dry saturated steam. This will occur if saturated steam contacts a surface with a higher temperature. Superheated steam and liquid water cannot coexist under thermodynamic equilibrium, as any additional heat simply evaporates more water and the steam will become sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygenation (medicine)
Oxygen saturation is the fraction of oxygen-saturated hemoglobin relative to total hemoglobin (unsaturated + saturated) in the blood. The human body requires and regulates a very precise and specific balance of oxygen in the blood. Normal arterial blood oxygen saturation levels in humans are 97–100 percent. If the level is below 90 percent, it is considered low and called hypoxemia. Arterial blood oxygen levels below 80 percent may compromise organ function, such as the brain and heart, and should be promptly addressed. Continued low oxygen levels may lead to respiratory or cardiac arrest. Oxygen therapy may be used to assist in raising blood oxygen levels. Oxygenation occurs when oxygen molecules () enter the tissues of the body. For example, blood is oxygenated in the lungs, where oxygen molecules travel from the air and into the blood. Oxygenation is commonly used to refer to medical oxygen saturation. Definition In medicine, oxygen saturation, commonly referred to as "sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated Zone
The phreatic zone, saturated zone, or zone of saturation, is the part of an aquifer, below the water table, in which relatively all pores and fractures are saturated with water. Above the water table is the unsaturated or vadose zone. The phreatic zone size, color, and depth may fluctuate with changes of season, and during wet and dry periods. Depending on the characteristics of soil particles, their packing and porosity, the boundary of a saturated zone can be stable or instable, exhibiting fingering patterns known as Saffman–Taylor instability. Predicting the onset of stable vs. unstable drainage fronts is of some importance in modelling phreatic zone boundaries. Dynamics of Drainage and Viscous Fingering in ''Transport in Porous Media'' ''Note that zones "behind" the dra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Kinetics
Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactions. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or a modifier (inhibitor or activator) might affect the rate. An enzyme (E) is typically a protein molecule that promotes a reaction of another molecule, its substrate (S). This binds to the active site of the enzyme to produce an enzyme-substrate complex ES, and is transformed into an enzyme-product complex EP and from there to product P, via a transition state ES*. The series of steps is known as the mechanism: : E + S ⇄ ES ⇄ ES* ⇄ EP ⇄ E + P This example assumes the simplest case of a reaction with one substrate and one product. Such cases exist: for example, a mutase such as phosphoglucomutase ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |