|
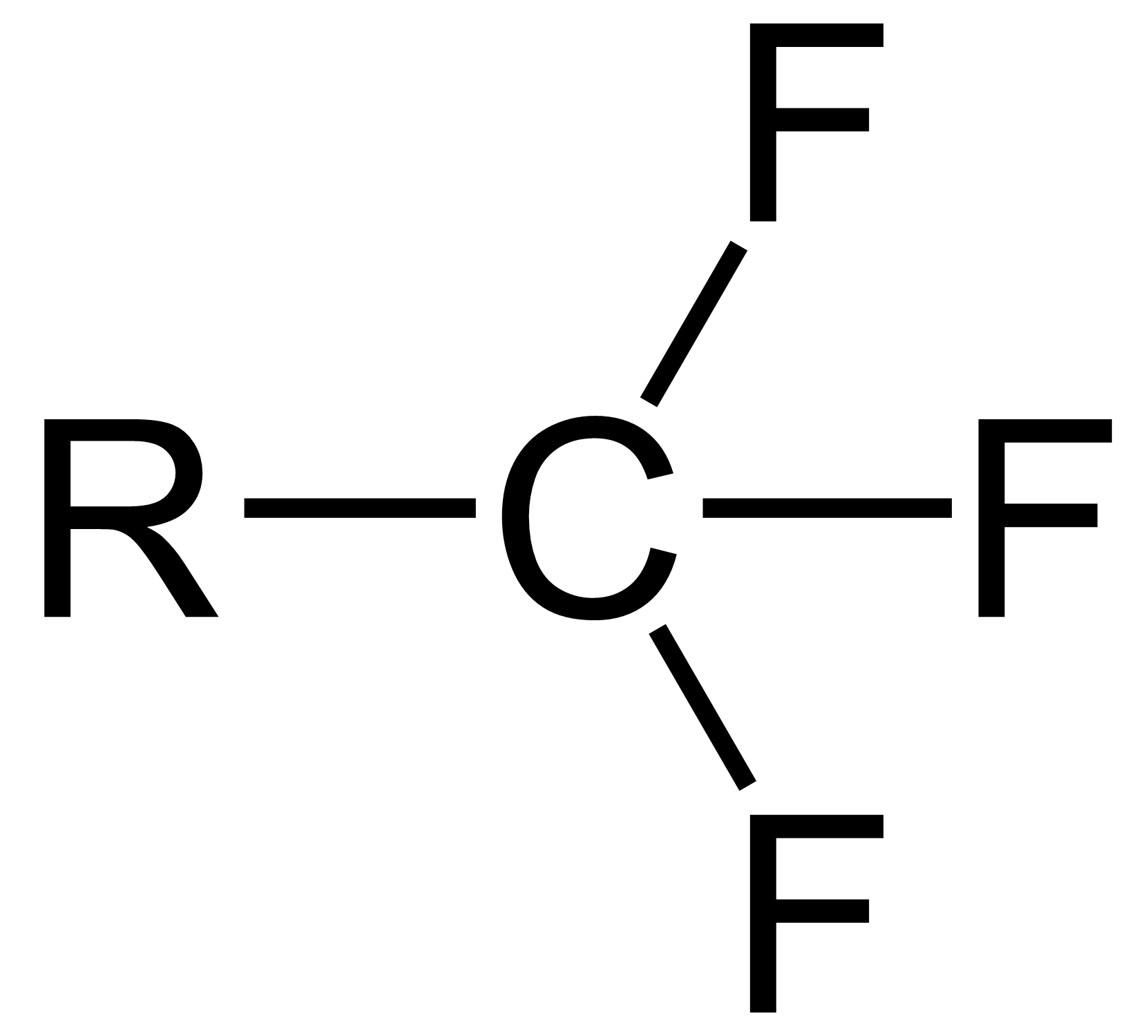
Trifluoromethyl
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoromethyl Copper
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorine
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/molKirsch, Peer ''Modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celecoxib
Celecoxib, sold under the brand name Celebrex among others, is a COX-2 inhibitor and nonsteroidal anti-inflammatory drug (NSAID). It is used to treat the pain and inflammation in osteoarthritis, acute pain in adults, rheumatoid arthritis, ankylosing spondylitis, painful menstruation, and juvenile rheumatoid arthritis. It may also be used to decrease the risk of colorectal adenomas in people with familial adenomatous polyposis. It is taken by mouth. Benefits are typically seen within an hour. Common side effects include abdominal pain, nausea, and diarrhea. Serious side effects may include heart attacks, strokes, gastrointestinal perforation, gastrointestinal bleeding, kidney failure, and anaphylaxis. Use is not recommended in people at high risk for heart disease. The risks are similar to other NSAIDs, such as ibuprofen and naproxen. Use in the later part of pregnancy or during breastfeeding is not recommended. Celecoxib was patented in 1993 and came into medical use in 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfoxaflor
Sulfoxaflor, also marketed as ''Isoclast'', is a systemic insecticide that acts as an insect neurotoxin. A pyridine and a trifluoromethyl compound, it is a member of a class of chemicals called sulfoximines, which act on the central nervous system of insects. Mechanism of action Sulfoxaflor is a systemic insecticide, acts as a neurotoxin to affected insects, and kills through contact or ingestion. Sulfoxaflor is classified for use against sap-feeding insects as a sulfoximine, which is a sub-group of insecticides that act as nicotinic acetylcholine receptor (nAChR) competitive modulators. Sulfoxaflor binds to nAChRs in place of acetylcholine. Sulfoxaflor binding causes uncontrolled nerve impulses resulting in muscle tremors followed by paralysis and death. Other nAChR competitive modulator sub-groups that bind differently on the receptor than sulfoximines include neonicotinoids, nicotine, and butenolides. Because sulfoxaflor binds much more strongly to insect neuron receptors than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoxetine
Fluoxetine, sold under the brand names Prozac and Sarafem, among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used for the treatment of major depressive disorder, obsessive–compulsive disorder (OCD), bulimia nervosa, panic disorder, and premenstrual dysphoric disorder. It is also approved for treatment of major depressive disorder in adolescents and children 8 years of age and over. It has also been used to treat premature ejaculation. Fluoxetine is taken by mouth. Common side effects include indigestion, trouble sleeping, sexual dysfunction, loss of appetite, dry mouth, and rash. Serious side effects include serotonin syndrome, mania, seizures, an increased risk of suicidal behavior in people under 25 years old, and an increased risk of bleeding. Antidepressant discontinuation syndrome is less likely to occur with fluoxetine than with other antidepressants, but it still happens in many cases. Fluoxetine taken during ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Efavirenz
Efavirenz (EFV), sold under the brand names Sustiva among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. It is sold both by itself and in combination as efavirenz/emtricitabine/tenofovir. It is taken by mouth. Common side effects include rash, nausea, headache, feeling tired, and trouble sleeping. Some of the rashes may be serious such as Stevens–Johnson syndrome. Other serious side effects include depression, thoughts of suicide, liver problems, and seizures. It is not safe for use during pregnancy. It is a non-nucleoside reverse transcriptase inhibitor (NNRTI) and works by blocking the function of reverse transcriptase. Efavirenz was approved for medical use in the United States in 1998, and in the European Union in 1999. It is on the World Health Organization's List of Essential Medicin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoroethanol
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethyl group, this alcohol exhibits a stronger acidic character compared to ethanol. Synthesis Trifluoroethanol is produced industrially by hydrogenation or the hydride reduction of derivatives of trifluoroacetic acid, such as the esters or acyl chloride. TFE can also be prepared by hydrogenolysis of compounds of generic formula CF3−CHOH−OR (where R is hydrogen or an alkyl group containing from one to eight carbon atoms), in the presence of a palladium containing catalyst deposited on activated charcoal. As a co-catalyst for this conversion tertiary aliphatic amines like triethylamine are commonly employed. Uses Trifluoroethanol is used as a solvent in organic chemistry. Oxidations of sulfur compounds using hydrogen peroxide are effective ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoroacetic Acid
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a structural analogue of acetic acid with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms and is a colorless liquid with a vinegar-like odor. TFA is a stronger acid than acetic acid, having an acid ionisation constant, ''K''a, that is approximately 34,000 times higher, as the highly electronegative fluorine atoms and consequent electron-withdrawing nature of the trifluoromethyl group weakens the oxygen-hydrogen bond (allowing for greater acidity) and stabilises the anionic conjugate base. TFA is widely used in organic chemistry for various purposes. Synthesis TFA is prepared industrially by the electrofluorination of acetyl chloride or acetic anhydride, followed by hydrolysis of the resulting trifluoroacetyl fluoride: : + 4 → + 3 + : + → + Where desired, this compound may be dried by addition of trifluoroacetic anhydride. An older route t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoromethanesulfonic Acid
Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for esterification. It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents. Synthesis Trifluoromethanesulfonic acid is produced industrially by electrochemical fluorination (ECF) of methanesulfonic acid: : CH3SO3H + 4 HF ->CF3SO2F + H2O + 3 H2 The resulting CF3SO2F is hydrolyzed, and the resulting triflate salt is reprotonated. Alternatively, trifluoromethanesulfonic acid arises by oxidation of trifluoromethylsulfenyl chloride: :CF3SCl + 2 Cl2 + 3 H2O -> CF3SO3H + 5 HCl Triflic acid is purified by distillation from triflic anhydride. Historical Trifluoromethanesulfonic acid was first synthesized in 1954 by Robert Haszeldine and Kidd by the following reaction: : Reactions As an acid In the laboratory, trifl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioisostere
In medicinal chemistry, bioisosteres are chemical substituents or groups with similar physical or chemical properties which produce broadly similar biological properties to another chemical compound. In drug design, the purpose of exchanging one bioisostere for another is to enhance the desired biological or physical properties of a compound without making significant changes in chemical structure. The main use of this term and its techniques are related to pharmaceutical sciences. Bioisosterism is used to reduce toxicity, change bioavailability, or modify the activity of the lead compound, and may alter the metabolism of the lead. Examples Classical bioisosteres Classical bioisosterism was originally formulated by James Moir and refined by Irving Langmuir as a response to the observation that different atoms with the same valence electron structure had similar biological properties. For example, the replacement of a hydrogen atom with a fluorine atom at a site of metabolism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |