|
Thermal Reservoir
A thermal reservoir, also thermal energy reservoir or thermal bath, is a thermodynamic system with a heat capacity so large that the temperature of the reservoir changes relatively little when a much more significant amount of heat is added or extracted. As a conceptual simplification, it effectively functions as an infinite pool of thermal energy at a given, constant temperature. Since it can act as a source and sink of heat, it is often also referred to as a heat reservoir or heat bath. Lakes, oceans and rivers often serve as thermal reservoirs in geophysical processes, such as the weather. In atmospheric science, large air masses in the atmosphere often function as thermal reservoirs. Since the temperature of a thermal reservoir does not change during the heat transfer, the change of entropy in the reservoir is :dS_=\frac. The microcanonical partition sum Z(E) of a heat bath of temperature has the property :Z(E+\Delta E)=Z(E)e^, where k_B is the Boltzmann constant. It th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic System
A thermodynamic system is a body of matter and/or radiation, confined in space by walls, with defined permeabilities, which separate it from its surroundings. The surroundings may include other thermodynamic systems, or physical systems that are not thermodynamic systems. A wall of a thermodynamic system may be purely notional, when it is described as being 'permeable' to all matter, all radiation, and all forces. A state of a thermodynamic system can be fully described in several different ways, by several different sets of thermodynamic state variables. A widely used distinction is between ''isolated'', ''closed'', and ''open'' thermodynamic systems. An isolated thermodynamic system has walls that are non-conductive of heat and perfectly reflective of all radiation, that are rigid and immovable, and that are impermeable to all forms of matter and all forces. (Some writers use the word 'closed' when here the word 'isolated' is being used.) A closed thermodynamic system is c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
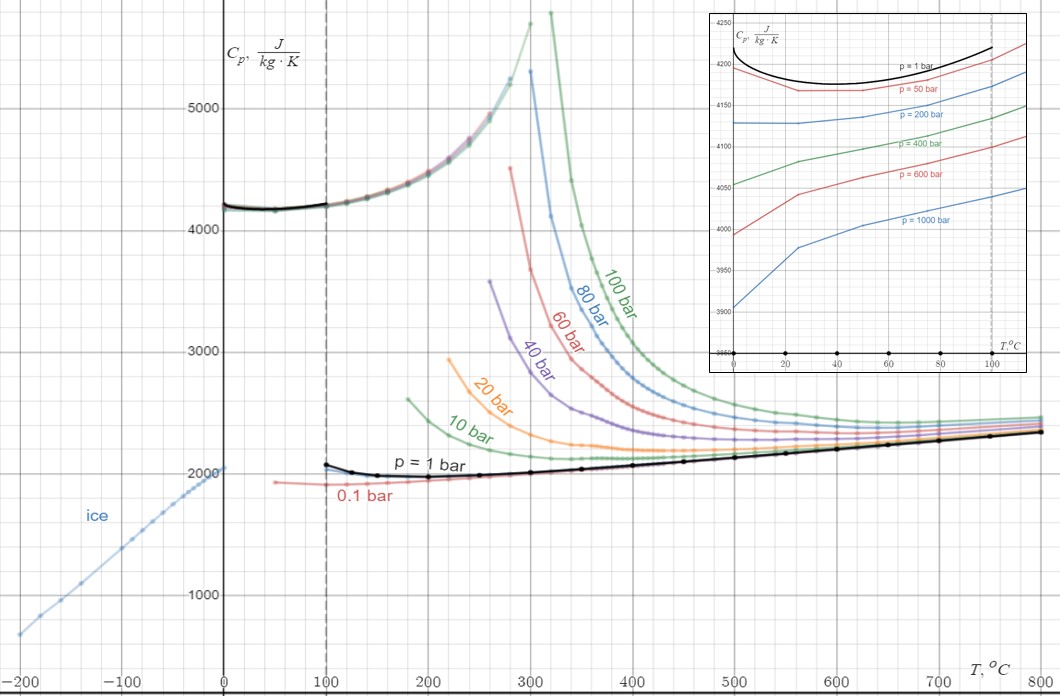
Heat Capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K). Heat capacity is an extensive property. The corresponding intensive property is the specific heat capacity, found by dividing the heat capacity of an object by its mass. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The volumetric heat capacity measures the heat capacity per volume. In architecture and civil engineering, the heat capacity of a building is often referred to as its thermal mass. Definition Basic definition The heat capacity of an object, denoted by C, is the limit : C = \lim_\frac, where \Delta Q is the amount of heat that must be added to the object (of mass ''M'') in order to raise its temperature by \Delta T. The value of this parameter usually varies considerably depending on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), the latter being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible to extract energy as heat from a body at that temperature. Temperature is important in all fields of natur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is also often used to refer to the thermal energy contained in a system as a component of its internal energy and that is reflected in the temperature of the system. For both uses of the term, heat is a form of energy. An example of formal vs. informal usage may be obtained from the right-hand photo, in which the metal bar is "conducting heat" from its hot end to its cold end, but if the metal bar is considered a thermodynamic system, then the energy flowing within the metal bar is called internal energy, not heat. The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of ''heat''. Another example of informal usage is the term '' heat content'', used despite the fact that p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Energy
The term "thermal energy" is used loosely in various contexts in physics and engineering. It can refer to several different well-defined physical concepts. These include the internal energy or enthalpy of a body of matter and radiation; heat, defined as a type of energy transfer (as is thermodynamic work); and the characteristic energy of a degree of freedom, k_T, in a system that is described in terms of its microscopic particulate constituents (where T denotes temperature and k_ denotes the Boltzmann constant). Relation to heat and internal energy In thermodynamics, heat is energy transferred to or from a thermodynamic system by mechanisms other than thermodynamic work or transfer of matter, such as conduction, radiation, and friction. Heat refers to a quantity transferred between systems, not to a property of any one system, or "contained" within it. On the other hand, internal energy and enthalpy are properties of a single system. Heat and work depend on the way in which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atmospheric Science
Atmospheric science is the study of the Atmosphere of Earth, Earth's atmosphere and its various inner-working physical processes. Meteorology includes atmospheric chemistry and atmospheric physics with a major focus on weather forecasting. Climatology is the study of atmospheric changes (both long and short-term) that define average climates and their change over time, due to both natural and anthropogenic climate change, climate variability. Aeronomy is the study of the upper layers of the atmosphere, where dissociation (chemistry), dissociation and ionization are important. Atmospheric science has been extended to the field of planetary science and the study of the atmospheres of the planets and natural satellites of the Solar System. Experimental instruments used in atmospheric science include satellites, rocketsondes, radiosondes, weather balloons, radars, and lasers. The term aerology (from Ancient Greek, Greek ἀήρ, ''aēr'', "air"; and -λογία, ''-logy, -logia'') is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Air Mass
In meteorology, an air mass is a volume of air defined by its temperature and humidity. Air masses cover many hundreds or thousands of square miles, and adapt to the characteristics of the surface below them. They are classified according to latitude and their continental or maritime source regions. Colder air masses are termed polar or arctic, while warmer air masses are deemed tropical. Continental and superior air masses are dry, while maritime and monsoon air masses are moist. Weather fronts separate air masses with different density (temperature or moisture) characteristics. Once an air mass moves away from its source region, underlying vegetation and body of water, water bodies can quickly modify its character. Classification schemes tackle an air mass' characteristics, as well as modification. Classification and notation The Tor Bergeron, Bergeron classification is the most widely accepted form of air mass classification, though others have produced more refined versions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
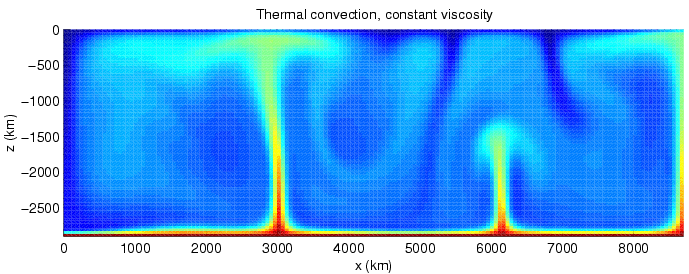
Heat Transfer
Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, Convection (heat transfer), thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species (mass transfer in the form of advection), either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system. Heat conduction, also called diffusion, is the direct microscopic exchanges of kinetic energy of particles (such as molecules) or quasiparticles (such as lattice waves) through the boundary between two systems. When an object is at a different temperature from another body or its surroundings, heat flows so that the body and the surroundings reach the same temperature, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change, and information systems including the transmission of information in telecommunication. The thermodynamic concept was referred to by Scottish scientist and engineer William Rankine in 1850 with the names ''thermodynamic function'' and ''heat-potential''. In 1865, German physicist Rudolf Clausius, one of the leading founders of the field of thermodynamics, defined it as the quotient of an infinitesimal amount of hea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microcanonical Ensemble
In statistical mechanics, the microcanonical ensemble is a statistical ensemble that represents the possible states of a mechanical system whose total energy is exactly specified. The system is assumed to be isolated in the sense that it cannot exchange energy or particles with its environment, so that (by conservation of energy) the energy of the system does not change with time. The primary macroscopic variables of the microcanonical ensemble are the total number of particles in the system (symbol: ), the system's volume (symbol: ), as well as the total energy in the system (symbol: ). Each of these is assumed to be constant in the ensemble. For this reason, the microcanonical ensemble is sometimes called the ensemble. In simple terms, the microcanonical ensemble is defined by assigning an equal probability to every microstate whose energy falls within a range centered at . All other microstates are given a probability of zero. Since the probabilities must add up to 1, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann Constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, and in Planck's law of black-body radiation and Boltzmann's entropy formula, and is used in calculating thermal noise in resistors. The Boltzmann constant has dimensions of energy divided by temperature, the same as entropy. It is named after the Austrian scientist Ludwig Boltzmann. As part of the 2019 redefinition of SI base units, the Boltzmann constant is one of the seven " defining constants" that have been given exact definitions. They are used in various combinations to define the seven SI base units. The Boltzmann constant is defined to be exactly . Roles of the Boltzmann constant Macroscopically, the ideal gas law states that, for an ideal gas, the product of pressure and volume is proportional to the product of amount of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann Factor
Factor, a Latin word meaning "who/which acts", may refer to: Commerce * Factor (agent), a person who acts for, notably a mercantile and colonial agent * Factor (Scotland), a person or firm managing a Scottish estate * Factors of production, such a factor is a resource used in the production of goods and services Science and technology Biology * Coagulation factors, substances essential for blood coagulation * Environmental factor, any abiotic or biotic factor that affects life * Enzyme, proteins that catalyze chemical reactions * Factor B, and factor D, peptides involved in the alternate pathway of immune system complement activation * Transcription factor, a protein that binds to specific DNA sequences Computer science and information technology * Factor (programming language), a concatenative stack-oriented programming language * Factor (Unix), a utility for factoring an integer into its prime factors * Factor, a substring, a subsequence of consecutive symbols in a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |