|
Tris(benzyltriazolylmethyl)amine
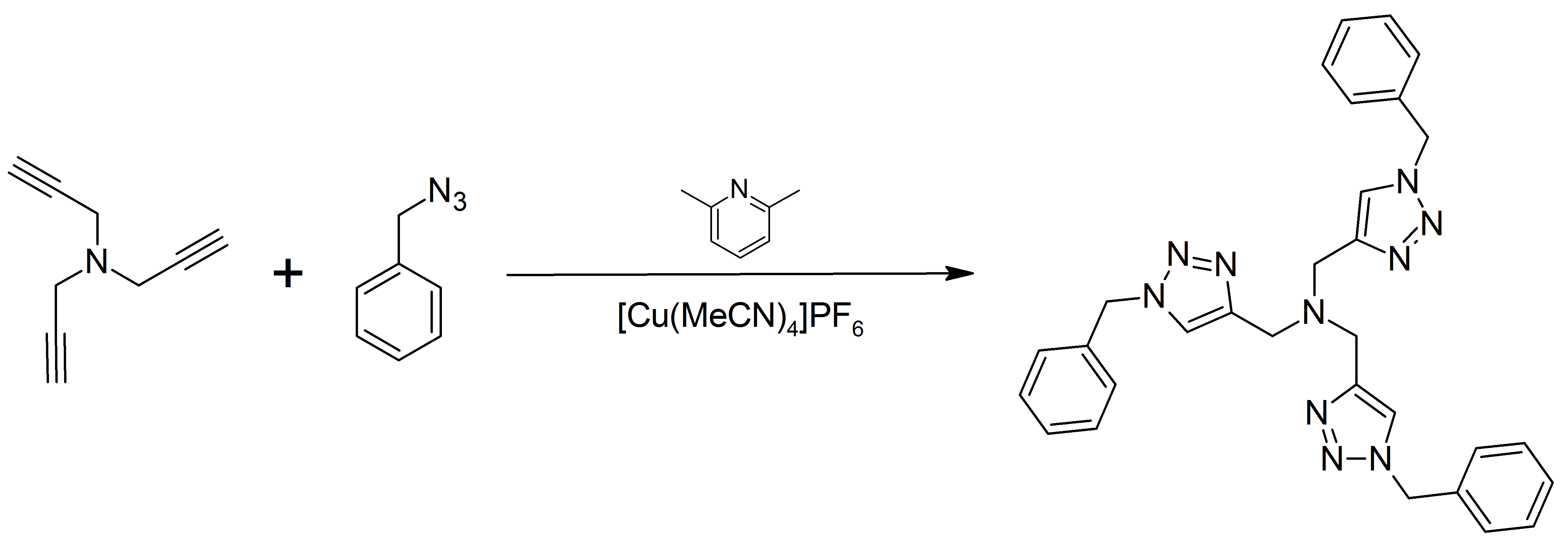
Tris((1-benzyl-4-triazolyl)methyl)amine (TBTA) is a tertiary amine containing the 1,2,3-triazole moiety. When used as a ligand, complexed to copper(I), it allows for quantitative, regioselective formal Huisgen 1,3-dipolar cycloadditions between alkynes and azides, in a variety of aqueous and organic solvents. It is believed that the ligand promotes catalysis through the stabilization of the copper(I)-oxidation state, while still allowing for the catalytic cycle of the CuAAC reaction to proceed. Single crystal X-ray diffraction of the Cu(I) complex of tris((1-benzyl-4-triazolyl)methyl)amine revealed an unusual dinuclear dication with one triazole unit bridging two metal centers, and is an effective catalyst for the 'click' cycloaddition reaction. The structure of the complex of TBTA with Cu(II) in the crystalline state is trigonal bipyramidal and can be reduced to the active 'click' catalyst form by sodium ascorbate, copper metal, or other reducing agents. In the literature, it has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Huisgen 1,3-dipolar Cycloaddition
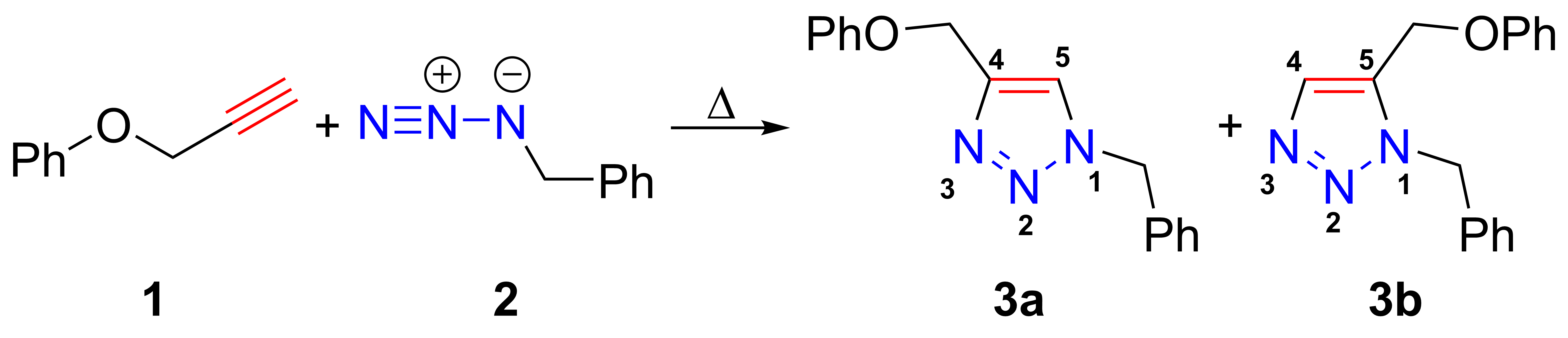
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction". In the reaction above azide 2 reacts neatly with alkyne 1 to afford the product triazole as a mixture of 1,4-adduct (3a) and 1,5-adduct (3b) at 98 °C in 18 hours. The standard 1,3-cycloaddition between an azide 1,3-dipole and an alkene as dipolarophile has largely been ignored due to lack of reactivity as a result of electron-poor olefins and elimination side reactions. Some success has been found with non-metal-catalyzed cycloadditions, such as the reactions using dipolarophiles that are electron-poor olefins or alkynes. Although azides are not the most reactive 1,3-dipole available for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substitue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant application of azides is as a propellant in air bags. Preparation Sodium azide is made industrially by the reaction of nitrous oxide, with sodium amide in liquid ammonia as solvent: : Many inorganic azides can be prepared directly or indirectly from sodium azide. For example, lead azide, used in detonators, may be prepared from the metathesis reaction between lead nitrate and sodium azide. An alternative route is direct reaction of the metal with silver azide dissolved in liquid ammonia. Some azides are produced by treating the carbonate salts with hydrazoic acid. Bonding Azide is isoelectronic with carbon dioxide , cyanate , nitrous oxide , nitronium ion and cyanogen fluoride NCF. Per valence bond theory, azide can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzyl Azide
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group. Nomenclature In IUPAC nomenclature, the prefix benzyl refers to a substituent, for example benzyl chloride or benzyl benzoate. Benzyl is not to be confused with phenyl with the formula . The term benzylic is used to describe the position of the first carbon bonded to a benzene or other aromatic ring. For example, is referred to as a "benzylic" carbocation. The benzyl free radical has the formula . The benzyl cation or phenylcarbenium ion is the carbocation with formula ; the benzyl anion or phenylmethanide ion is the carbanion with the formula . None of these species can be formed in significant amounts in the solution phase under normal conditions, but they are useful referents for discussion of reaction mechanisms and may exist as reactive intermediates. Abbreviations The abbreviation "Bn" denotes benzyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem
Chem may refer to: * Chemistry practical waali mam * Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" *Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel * CHEM-DT, a Canadian television channel See also *Chemo (other) Chemo is a prefix meaning ''chemical'' and commonly used as an abbreviation for chemotherapy. Chemo may also refer to: People * Chemo (musician), an English musician now known as Forest DLG * Chemo Soto, a Puerto Rican politician * José del Sol ... * Kemi, a place in Finland {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Preparation Of TBTA
Preparation may refer to: * Preparation (dental), the method by which a tooth is prepared when removing decay and designing a form that will provide adequate retention for a dental restoration * Preparation (music), treatment of dissonance in tonal music * Preparation, Iowa, a ghost town * Preparation time, time to prepare speeches in policy debate * ''The Preparation'', a 2017 South Korean film * ''Preparations'' (album), a 2007 album by Prefuse 73 * Prepared dosage form * Prepared drug * Prepared food * Prepared supplement * Special modifications to instruments, see **Prepared piano **Prepared guitar *Fossil preparation See also * Preparation H, popular hemorrhoids medicine * Preparation for the Gospel, early Christian book * * Prep (other) * Preparationism * Prepare (other) * Preparedness (other) Preparedness refers to actions that are taken as precautionary measures in the face of potential disasters Preparedness may also refer to: * Emergency ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagents For Organic Chemistry
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, '' catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |