|
Transient Kinetic Isotope Fractionation
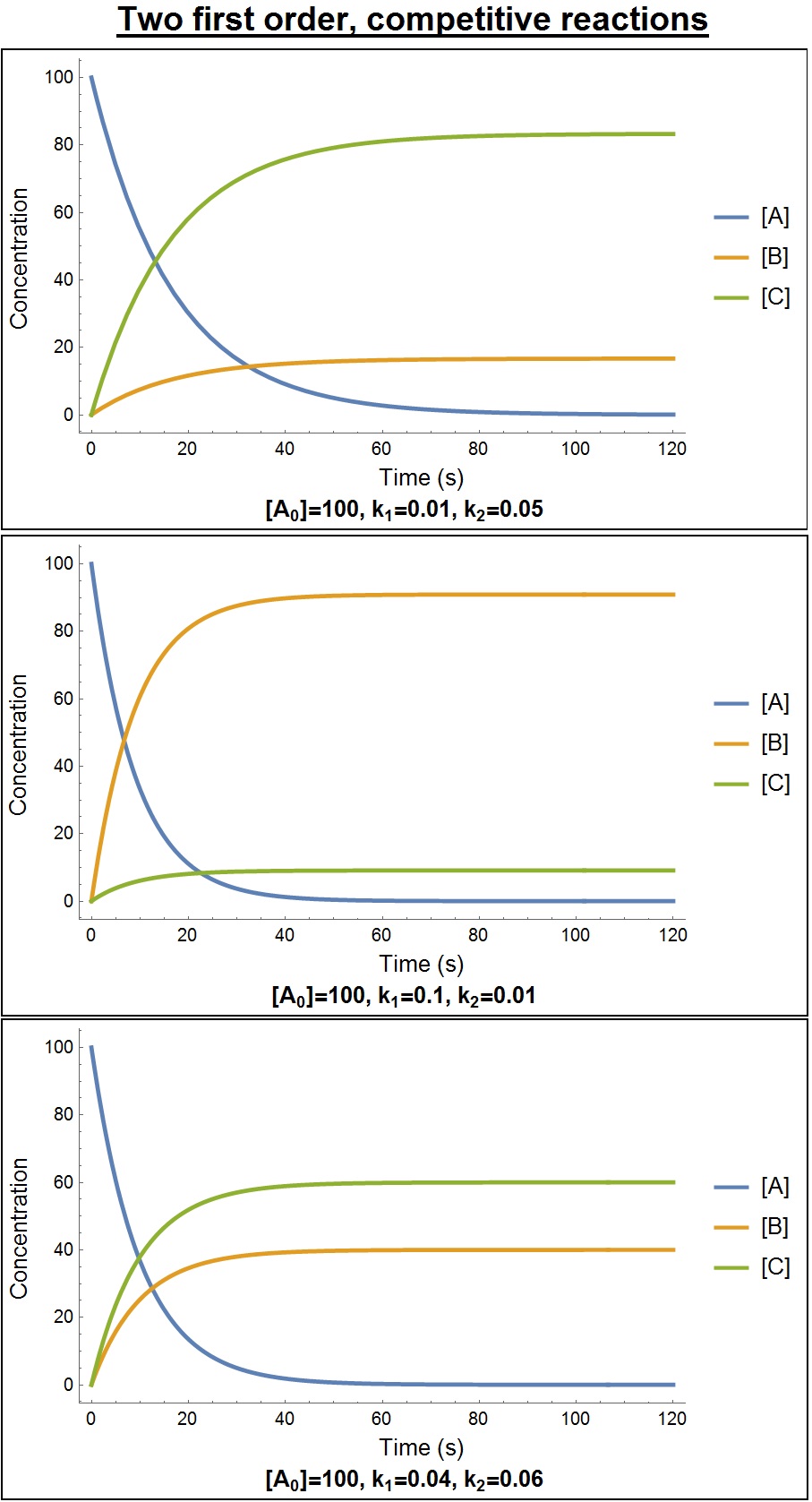
Transient kinetic isotope effects (or fractionation) occur when the reaction leading to isotope fractionation does not follow pure first-order kinetics (FOK) and therefore isotopic effects cannot be described with the classical equilibrium fractionation equations or with steady-state kinetic fractionation equations (also known as the Rayleigh equation). In these instances, the general equations for biochemical isotope kinetics (GEBIK) and the general equations for biochemical isotope fractionation (GEBIF) can be used. The GEBIK and GEBIF equations are the most generalized approach to describe isotopic effects in any chemical, catalytic reaction and biochemical reactions because they can describe isotopic effects in equilibrium reactions, kinetic chemical reactions and kinetic biochemical reactions. In the latter two cases, they can describe both stationary and non-stationary fractionation (i.e., variable and inverse fractionation). In general, isotopic effects depend on the num ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemical Reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
First-order Kinetics
In chemistry, the rate equation (also known as the rate law or empirical differential rate equation) is an empirical differential mathematical expression for the reaction rate of a given reaction in terms of concentrations of chemical species and constant parameters (normally rate coefficients and partial orders of reaction) only. For many reactions, the initial rate is given by a power law such as :v_0\; =\; k mathrmx mathrmy where and are the molar concentrations of the species and usually in moles per liter (molarity, ). The exponents and are the partial ''orders of reaction'' for and , respectively, and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The order of reaction is a number which quantifies the degree to which the rate of a chemical reaction depends on concentrations of the reactants. In other words, the order of reaction is the exponent to which the concentrati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Equilibrium Fractionation
Equilibrium isotope fractionation is the partial separation of isotopes between two or more substances in chemical equilibrium. Equilibrium fractionation is strongest at low temperatures, and (along with kinetic isotope effects) forms the basis of the most widely used isotopic paleothermometers (or climate proxies): D/H and 18O/16O records from ice cores, and 18O/16O records from calcium carbonate. It is thus important for the construction of geologic temperature records. Isotopic fractionations attributed to equilibrium processes have been observed in many elements, from hydrogen ( D/H) to uranium ( 238U/235U). In general, the light elements (especially hydrogen, boron, carbon, nitrogen, oxygen and sulfur) are most susceptible to fractionation, and their isotopes tend to be separated to a greater degree than heavier elements. Definition Most equilibrium fractionations are thought to result from the reduction in vibrational energy (especially zero-point energy) when a more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
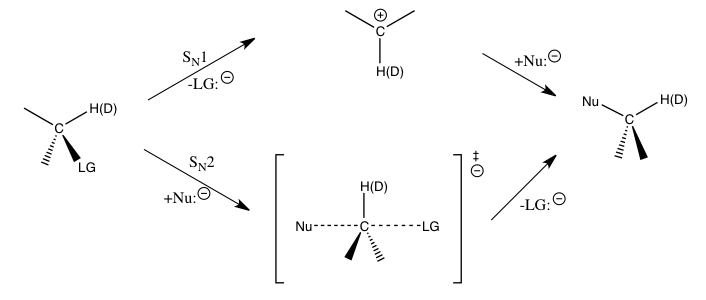
Kinetic Isotope Effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for the reactions involving the light (''k'') and the heavy (''k'') isotopically substituted reactants ( isotopologues): KIE = ''k/k''. This change in reaction rate is a quantum effect that occurs mainly because heavier isotopologues have lower vibrational frequencies than their lighter counterparts. In most cases, this implies a greater energy input needed for heavier isotopologues to reach the transition state (or, in rare cases, dissociation limit), and therefore, a slower reaction rate. The study of KIEs can help elucidate reaction mechanisms, and is occasionally exploited in drug development to improve unfavorable pharmacokinetics by protecting metabolically vulnerable C-H bonds. Background KIE is considered one of the most essential ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Rate Equation
In chemistry, the rate equation (also known as the rate law or empirical differential rate equation) is an Empirical relationship, empirical Differential equation, differential Expression (mathematics), mathematical expression for the reaction rate of a given reaction in terms of concentrations of chemical species and constant parameters (normally rate coefficients and partial orders of reaction) only. For many reactions, the initial rate is given by a power law such as :v_0\; =\; k[\mathrm]^x[\mathrm]^y where and are the molar concentrations of the species and usually in Mole (unit), moles per liter (molarity, ). The exponents and are the partial ''orders of reaction'' for and , respectively, and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The order of reaction is a number which quantifies the degree to which the rate of a chemical reaction depends on concentrations of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Enzymatic Reaction
Enzyme catalysis is the increase in the rate of a process by an "enzyme", a biological molecule. Most enzymes are proteins, and most such processes are chemical reactions. Within the enzyme, generally catalysis occurs at a localized site, called the active site. Most enzymes are made predominantly of proteins, either a single protein chain or many such chains in a multi-subunit complex. Enzymes often also incorporate non-protein components, such as metal ions or specialized organic molecules known as cofactor (e.g. adenosine triphosphate). Many cofactors are vitamins, and their role as vitamins is directly linked to their use in the catalysis of biological process within metabolism. Catalysis of biochemical reactions in the cell is vital since many but not all metabolically essential reactions have very low rates when uncatalysed. One driver of protein evolution is the optimization of such catalytic activities, although only the most crucial enzymes operate near catalytic e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Michaelis–Menten Kinetics
In biochemistry, Michaelis–Menten kinetics, named after Leonor Michaelis and Maud Menten, is the simplest case of enzyme kinetics, applied to enzyme-catalysed reactions involving the transformation of one substrate into one product. It takes the form of a differential equation describing the reaction rate v (rate of formation of product (biology), product P, with concentration p) as a function of a, the concentration of the Enzyme substrate (biology), substrate A (using the symbols recommended by the IUBMB). Its formula is given by the Michaelis–Menten equation: : v = \frac = \frac V, which is often written as V_\max, represents the limiting rate approached by the system at saturating substrate concentration for a given enzyme concentration. The Michaelis constant K_\mathrm has units of concentration, and for a given reaction is equal to the concentration of substrate at which the reaction rate is half of V. Biochemical reactions involving a single substrate are often ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more common H has no neutrons. The name ''deuterium'' comes from Greek '' deuteros'', meaning "second". American chemist Harold Urey discovered deuterium in 1931. Urey and others produced samples of heavy water in which the H had been highly concentrated. The discovery of deuterium won Urey a Nobel Prize in 1934. Nearly all deuterium found in nature was synthesized in the Big Bang 13.8 billion years ago, forming the primordial ratio of H to H (~26 deuterium nuclei per 10 hydrogen nuclei). Deuterium is subsequently produced by the slow stellar proton–proton chain, but rapidly destroyed by exothermic fusion reactions. The deuterium–deuterium reaction has the second-lowest energy threshold, and is the most astrophysically acce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national List of chemistry societies, chemistry societies, national Academy of Sciences, academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrous Oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or factitious air, among others, is a chemical compound, an Nitrogen oxide, oxide of nitrogen with the Chemical formula, formula . At room temperature, it is a colourless Flammability#Definitions, non-flammable gas, and has a slightly sweet scent and taste. At elevated temperatures, nitrous oxide is a powerful Oxidising agent, oxidiser similar to molecular oxygen. Nitrous oxide has significant Nitrous oxide (medication), medical uses, especially in surgery and dentistry, for its Anesthesia, anaesthetic and Analgesic, pain-reducing effects, and it is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. Its colloquial name, "laughing gas", coined by Humphry Davy, describes the Euphoria, euphoric effects upon inhaling it, which cause it to be used as a recreational drug inducing a brief "Dissociative, high". When abused chronically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Steady State (chemistry)
In chemistry, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system (compare mass balance). A simple example of such a system is the case of a bathtub with the tap running but with the drain unplugged: after a certain time, the water flows in and out at the same rate, so the water level (the state variable Volume) stabilizes and the system is in a steady state. The steady state concept is different from chemical equilibrium. Although both may create a situation where a concentration does not change, in a system at chemical equilibrium, the net reaction rate is zero ( products transform into reactants at the same rate as reactants transform into products), while no such limitation exists in the steady state concept. Indeed, there does not have to be a reaction a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Equilibrium Fractionation
Equilibrium isotope fractionation is the partial separation of isotopes between two or more substances in chemical equilibrium. Equilibrium fractionation is strongest at low temperatures, and (along with kinetic isotope effects) forms the basis of the most widely used isotopic paleothermometers (or climate proxies): D/H and 18O/16O records from ice cores, and 18O/16O records from calcium carbonate. It is thus important for the construction of geologic temperature records. Isotopic fractionations attributed to equilibrium processes have been observed in many elements, from hydrogen ( D/H) to uranium ( 238U/235U). In general, the light elements (especially hydrogen, boron, carbon, nitrogen, oxygen and sulfur) are most susceptible to fractionation, and their isotopes tend to be separated to a greater degree than heavier elements. Definition Most equilibrium fractionations are thought to result from the reduction in vibrational energy (especially zero-point energy) when a more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |