|
Thioglycerol
3-Mercaptopropane-1,2-diol, also known as thioglycerol, is a chemical compound and thiol that is used as a matrix in fast atom bombardment mass spectrometry and liquid secondary ion mass spectrometry. See also * Glycerol * Mercaptoethanol 2-Mercaptoethanol (also β-mercaptoethanol, BME, 2BME, 2-ME or β-met) is the chemical compound with the chemical formula, formula HOCH2CH2SH. ME or βME, as it is commonly abbreviated, is used to reduce disulfide bonds and can act as a biological ... References {{DEFAULTSORT:Mercaptopropane-1,2-diol, 3- Solvents Thiols Vicinal diols Mass spectrometry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
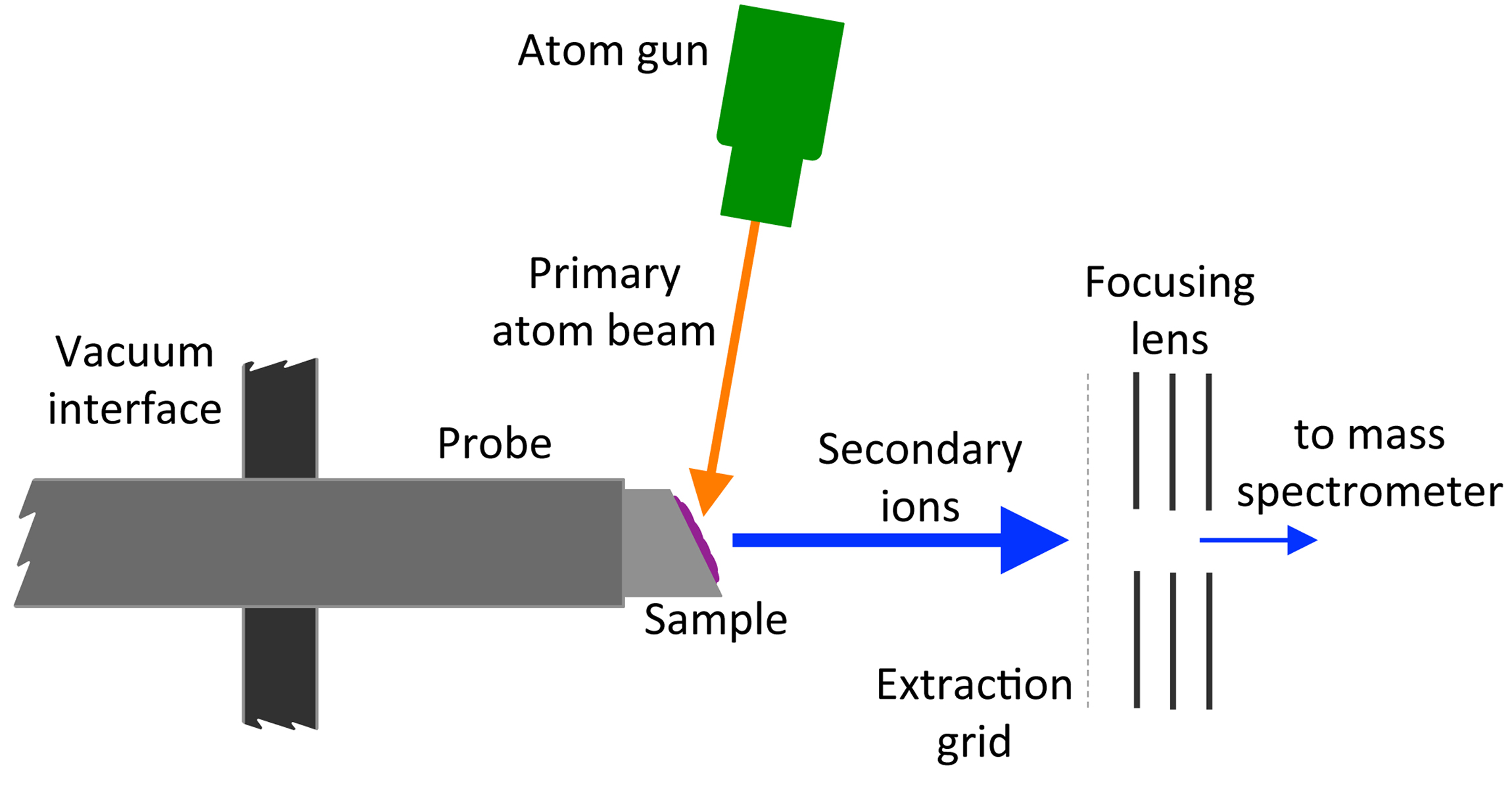
Fast Atom Bombardment
Fast atom bombardment (FAB) is an ionization technique used in mass spectrometry in which a beam of high energy atoms strikes a surface to create ions. It was developed by Michael Barber (chemist), Michael Barber at the University of Manchester in 1980. When a beam of high energy ions is used instead of atoms (as in secondary ion mass spectrometry), the method is known as liquid secondary ion mass spectrometry (LSIMS). In FAB and LSIMS, the material to be analyzed is mixed with a non-volatile chemical protection environment, called a Matrix Isolation, matrix, and is bombarded under vacuum with a high energy (4000 to 10,000 electron volts) atomic beam. The atoms are typically from an inert gas such as argon or xenon. Common matrices include glycerol, thioglycerol, 3-nitrobenzyl alcohol (3-NBA), 18-crown-6 ether, 2-nitrophenyloctyl ether, sulfolane, diethanolamine, and triethanolamine. This technique is similar to secondary ion mass spectrometry and plasma desorption mass spectrometry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic, cabbage or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the smell of natural gas is due to the smell of the thiol used as the odorant. Nomenclature Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionization, ionized, for example by bombarding it with a Electron ionization, beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragmen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Ion Mass Spectrometry
Secondary-ion mass spectrometry (SIMS) is a technique used to analyze the composition of solid surfaces and thin films by sputtering the surface of the specimen with a focused primary ion beam and collecting and analyzing ejected secondary ions. The mass/charge ratios of these secondary ions are measured with a mass spectrometer to determine the elemental, isotopic, or molecular composition of the surface to a depth of 1 to 2 nm. Due to the large variation in ionization probabilities among elements sputtered from different materials, comparison against well-calibrated standards is necessary to achieve accurate quantitative results. SIMS is the most sensitive surface analysis technique, with elemental detection limits ranging from parts per million to parts per billion. History In 1910, British physicist J. J. Thomson observed a release of positive ions and neutral atoms from a solid surface induced by ion bombardment. Improved vacuum pump technology in the 1940s enabled th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol () is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is Hygroscopy, hygroscopic in nature. Modern use of the word glycerine (alternatively spelled glycerin) refers to commercial preparations of less than 100% purity, typically 95% glycerol. Structure Although chirality, achiral, glycerol is prochirality, prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the Glycerophospholipid#Nomenclature and stereochemistry, stereospecific numbering labels the molecule with a ''sn''- prefix before the stem name of the molecule. Production Natural sources Glycerol is generally obtained from plant and animal sources where it occurs in triglycerides, est ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercaptoethanol
2-Mercaptoethanol (also β-mercaptoethanol, BME, 2BME, 2-ME or β-met) is the chemical compound with the chemical formula, formula HOCH2CH2SH. ME or βME, as it is commonly abbreviated, is used to reduce disulfide bonds and can act as a biological antioxidant by scavenging hydroxyl radicals (amongst others). It is widely used because the hydroxyl group confers solubility in water and lowers the volatility. Due to its diminished vapor pressure, its odor, while unpleasant, is less objectionable than related thiols. Production 2-Mercaptoethanol is manufactured industrially by the reaction of ethylene oxide with hydrogen sulfide. Thiodiglycol and various zeolites catalyze the reaction. : Reactions 2-Mercaptoethanol reacts with aldehydes and ketones to give the corresponding oxathiolanes. This makes 2-mercaptoethanol useful as a protecting group, giving a derivative whose stability is between that of a dioxolane and a 1,3-Dithiolane, dithiolane. : Applications Reducing proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvents
A solvent (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules, and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical syntheses and purification processes Some petrochemical solvents are highly toxic and emit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiols
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic, cabbage or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the smell of natural gas is due to the smell of the thiol used as the odorant. Nomenclature Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vicinal Diols of changed physical/molecular structure at the boundary of many liquids and a barrier has been described as the interfacial layer or the vicinal layer.
{{disambig ...
Vicinal ''("In the neighbourhood of")'' is a general adjective used as a standard, specific, technical descriptor in a range of contexts, including: * Vicinal (chemistry), stands for any two functional groups bonded to two adjacent atoms. * Vicinal tramway or ''Buurtspoor'', a system of narrow gauge tramways or local railways in Belgium. * In materials science, a "vicinal substrate" is a thin-film substrate whose surface normal deviates slightly from a major crystallographic axis. * In physics, the exclusion zone An exclusion zone is a geographic area in which specific activities are prohibited by an authority. The United States Department of Defense defines an exclusion zone is a territory where an authority prohibits specific activities in a specific g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |