|
Tantalum Pentoxide
Tantalum pentoxide, also known as tantalum(V) oxide, is the inorganic compound with the formula . It is a white solid that is insoluble in all solvents but is attacked by strong bases and hydrofluoric acid. is an inert material with a high refractive index and low absorption (i.e. colourless), which makes it useful for coatings. It is also extensively used in the production of capacitors, due to its high dielectric constant. Preparation Occurrence Tantalum occurs in the minerals tantalite and columbite (columbium being an archaic name for niobium), which occur in pegmatites, an igneous rock formation. Mixtures of columbite and tantalite are called coltan. Tantalum was discovered in Tantalite in 1802 by Anders Gustaf Ekeberg at Ytterby, Sweden, and Kimoto, Finland. The minerals microlite and pyrochlore contain approximately 70% and 10% Ta, respectively. Refining Tantalum ores often contain significant amounts of niobium, which is itself a valuable metal. As such, both metals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral Acid
A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds, as opposed to organic acids which are acidic, organic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water. Characteristics Commonly used mineral acids are sulfuric acid (H2SO4), hydrochloric acid (HCl) and nitric acid (HNO3); these are also known as bench acids. Mineral acids range from superacids (such as perchloric acid) to very weak ones (such as boric acid). Mineral acids tend to be very soluble in water and insoluble in organic solvents. Mineral acids are used in many sectors of the chemical industry as feedstocks for the synthesis of other chemicals, both organic and inorganic. Large quantities of these acids—especially sulfuric acid, nitric acid, and hydrochloric acid—are manufactured for commercial use in large plants. Mineral acids are also used directly for their corrosive properties. For example, a dilute solution of hydrochl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrochlore
Pyrochlore () is a mineral group of the niobium end member of the pyrochlore supergroup. Pyrochlore is also a term for the crystal structure ''F''dm. The name is from the Greek , ''fire'', and , ''green'' because it typically turns green on ignition in classic blowpipe analysis. Mineral The general formula, (where A and B are metals), represent a family of phases isostructural to the mineral pyrochlore. Pyrochlores are an important class of materials in diverse technological applications such as luminescence, ionic conductivity, nuclear waste immobilization, high-temperature thermal barrier coatings, automobile exhaust gas control, catalysts, solid oxide fuel cell, ionic/electrical conductors etc. The mineral is associated with the metasomatic end stages of magmatic intrusions. Pyrochlore crystals are usually well-formed (euhedral), occurring usually as octahedra of a yellowish or brownish color and resinous luster. It is commonly metamict due to radiation damage from includ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Isobutyl Ketone
Methyl isobutyl ketone (MIBK, 4-methylpentan-2-one) is an organic compound with the structural formula, condensed chemical formula (CH3)2CHCH2C(O)CH3. This ketone is a colourless liquid that is used as a solvent for gums, resins, paints, varnishes, lacquers, and nitrocellulose. Production At laboratory scale, MIBK can be produced via a three-step process using acetone as the starting material. Self-condensation, a type of aldol reaction, produces diacetone alcohol, which readily dehydration (chemistry), dehydrates to give 4-methylpent-3-en-2-one (commonly, mesityl oxide). Mesityl oxide is then hydrogenation, hydrogenated to give MIBK. : Industrially, these three steps are combined. Acetone is treated with a strongly acidic, palladium catalyst-doped Ion-exchange resin, cation exchange resin under medium pressure of hydrogen. Several million kilograms are produced annually.Stylianos Sifniades, Alan B. Levy, "Acetone" in ''Ullmann’s Encyclopedia of Industrial Chemistry'', Wiley-VCH, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of cyclohexanone assume a pale yellow color. Cyclohexanone is slightly soluble in water and miscible with common organic solvents. Millions of tonnes are produced annually, mainly as a precursor to nylon. History and synthesis The compound was discovered by in 1888 among the products of AC electrolysis of slightly acidified water solutions of phenol. He named it hydrophenoketone and correctly suggested that phenol was first hydrogenated by electrolytic hydrogen to cyclohexanol, which he wasn't able to isolate, and then oxidized by electrolytic oxygen. Laboratory synthesis Cyclohexanone can be prepared from cyclohexanol by oxidation with chromium trioxide ( Jones oxidation). An alternative method utilizes the safer and more readily avai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Solvents
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for Chemical polarity#Polarity of molecules, polar molecules, and the most common solvent used by living things; all the ions and proteins in a Cell (biology), cell are dissolved in water within the cell. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for Organic compound, organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents (D-limonene, citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid–liquid Extraction
Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubility, solubilities in two different Miscibility, immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more Chemical species, species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration (lower free energy). The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. Liquid–liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as Mixe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water would be represented as . The word ''aqueous'' (which comes from ''aqua'') means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since water is frequently used as the solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified. A ''non-aqueous solution'' is a solution in which the solvent is a liquid, but is not water. Characteristics Substances that are '' hydrophobic'' ('water-fearing') do not dissolve well in water, whereas those that are '' hydrophilic'' ('water-friendly') do. An example of a hydrophilic substance is sodium chloride. In an aqueous solution the hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese(II) Fluoride
Manganese(II) fluoride is the chemical compound composed of manganese and fluoride with the formula MnF2. It is a light pink solid, the light pink color being characteristic for manganese(II) compounds. It is made by treating manganese and diverse compounds of manganese(II) in hydrofluoric acid. Like some other metal difluorides, MnF2 crystallizes in the rutile structure, which features octahedral Mn centers. Uses MnF2 is used in the manufacture of special kinds of glass and laser A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...s. It is a canonical example of uniaxial antiferromagnet (with Neel temperature of 68 K) which has been experimentally studied since early on. References Manganese(II) compounds Fluorides Metal halides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Fluoride
Iron(II) fluoride or ferrous fluoride is an inorganic compound with the molecular formula FeF2. It forms a tetrahydrate FeF2·4H2O that is often referred to by the same names. The anhydrous and hydrated forms are white crystalline solids.Dale L. Perry (1995),Handbook of Inorganic Compounds, page 167. CRC Press. Structure and bonding Anhydrous FeF2 adopts the TiO2 rutile structure. As such, the iron cations are octahedral and fluoride anions are trigonal planar. The tetrahydrate can exist in two structures, or polymorphs. One form is rhombohedral and the other is hexagonal, the former having a disorder. Like most fluoride compounds, the anhydrous and hydrated forms of iron(II) fluoride feature high spin metal center. Low temperature neutron diffraction studies show that the FeF2 is antiferromagnetic. Heat capacity measurements reveal an event at 78.3 K corresponding to ordering of antiferromagnetic state. Selected physical properties FeF2 sublimes between 958 and 1178 K. U ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Heptafluorotantalate
Potassium heptafluorotantalate is an inorganic compound with the formula K2 aF7 It is the potassium salt of the heptafluorotantalate anion aF7sup>2−. This white, water-soluble solid is an intermediate in the purification of tantalum from its ores and is the precursor to the metal. Preparation Industrial Potassium heptafluorotantalate is an intermediate in the industrial production of metallic tantalum. Its production involves leaching tantalum ores, such as columbite and tantalite, with hydrofluoric acid and sulfuric acid to produce the water-soluble hydrogen heptafluorotantalate. : Ta2O5 + 14 HF → 2 H2 aF7 + 5 H2O This solution is subjected to a number of liquid-liquid extraction steps to remove metallic impurities (most importantly niobium) before being treated with potassium fluoride to produce K2 aF7 : H2 aF7+ 2 KF → K2 aF7+ 2 HF Lab-scale Hydrofluoric acid is both corrosive and toxic, making it unappealing to work with; as such a number of alternative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
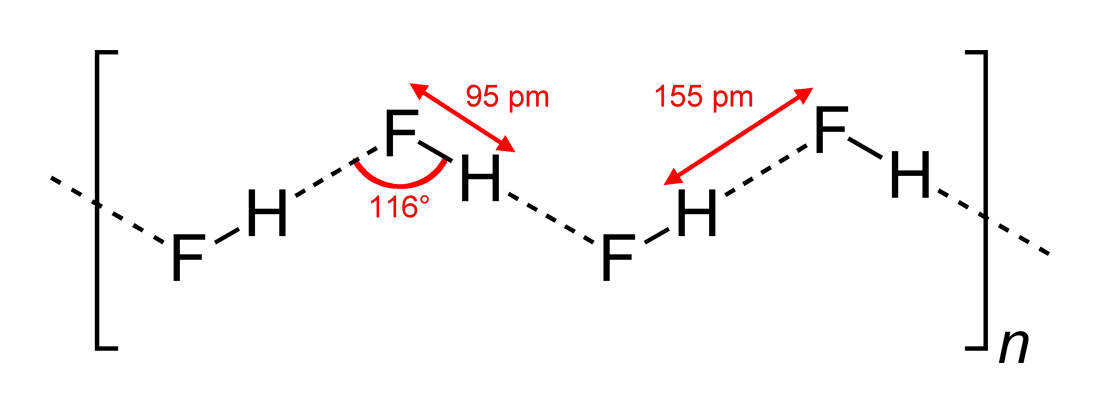
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula . It is a colorless, odorless, and Viscosity, viscous liquid that is Miscibility, miscible with water. Pure sulfuric acid does not occur naturally due to its Dehydration reaction, strong affinity to water vapor; it is Hygroscopy, hygroscopic and readily absorbs water vapor from the Atmosphere of Earth, air. Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is releas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |