|
Standard Atomic Weight
The standard atomic weight of a chemical element (symbol ''A''r°(E) for element "E") is the weighted arithmetic mean of the relative isotopic masses of all isotopes of that element weighted by each isotope's abundance on Earth. For example, isotope 63Cu (''A''r = 62.929) constitutes 69% of the copper on Earth, the rest being 65Cu (''A''r = 64.927), so :A_\text\text(_\text\text)=0.69\times62.929+0.31\times64.927=63.55. Because relative isotopic masses are dimensionless quantities, this weighted mean is also dimensionless. It can be converted into a measure of mass (with dimension ) by multiplying it with the dalton, also known as the atomic mass constant. Among various variants of the notion of atomic weight (''A''r, also known as '' relative atomic mass'') used by scientists, the standard atomic weight is the most common and practical. The standard atomic weight of each chemical element is determined and published by the Commission on Isotopic Abundances and Atomic Weights ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Mass
The atomic mass (''m''a or ''m'') is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1 Da is defined as of the mass of a free carbon-12 atom at rest in its ground state. The protons and neutrons of the nucleus account for nearly all of the total mass of atoms, with the electrons and nuclear binding energy making minor contributions. Thus, the numeric value of the atomic mass when expressed in daltons has nearly the same value as the mass number. Conversion between mass in kilograms and mass in daltons can be done using the atomic mass constant m_= = 1\ \rm . The formula used for conversion is: :1\ = m_ = 1.660\ 539\ 066\ 60(50)\times 10^\ \mathrm , where M_ is the molar mass constant, N_ is the Avogadro constant, and M(^\mathrm) is the experimentally determined molar mass of carbon-12. The relative isotopic mass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Earth's Atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing for liquid water to exist on the Earth's surface, absorbing ultraviolet solar radiation, warming the surface through heat retention ( greenhouse effect), and reducing temperature extremes between day and night (the diurnal temperature variation). By mole fraction (i.e., by number of molecules), dry air contains 78.08% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide, and small amounts of other gases. Air also contains a variable amount of water vapor, on average around 1% at sea level, and 0.4% over the entire atmosphere. Air composition, temperature, and atmospheric pressure vary with altitude. Within the atmosphere, air suitable for use in photosynthesis by terrestrial plants and breathing of terrestrial animals is fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Astrophysical Journal
''The Astrophysical Journal'', often abbreviated ''ApJ'' (pronounced "ap jay") in references and speech, is a peer-reviewed scientific journal of astrophysics and astronomy, established in 1895 by American astronomers George Ellery Hale and James Edward Keeler. The journal discontinued its print edition and became an electronic-only journal in 2015. Since 1953 ''The Astrophysical Journal Supplement Series'' (''ApJS'') has been published in conjunction with ''The Astrophysical Journal'', with generally longer articles to supplement the material in the journal. It publishes six volumes per year, with two 280-page issues per volume. ''The Astrophysical Journal Letters'' (''ApJL''), established in 1967 by Subrahmanyan Chandrasekhar as Part 2 of ''The Astrophysical Journal'', is now a separate journal focusing on the rapid publication of high-impact astronomical research. The three journals were published by the University of Chicago Press for the American Astronomical Society ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
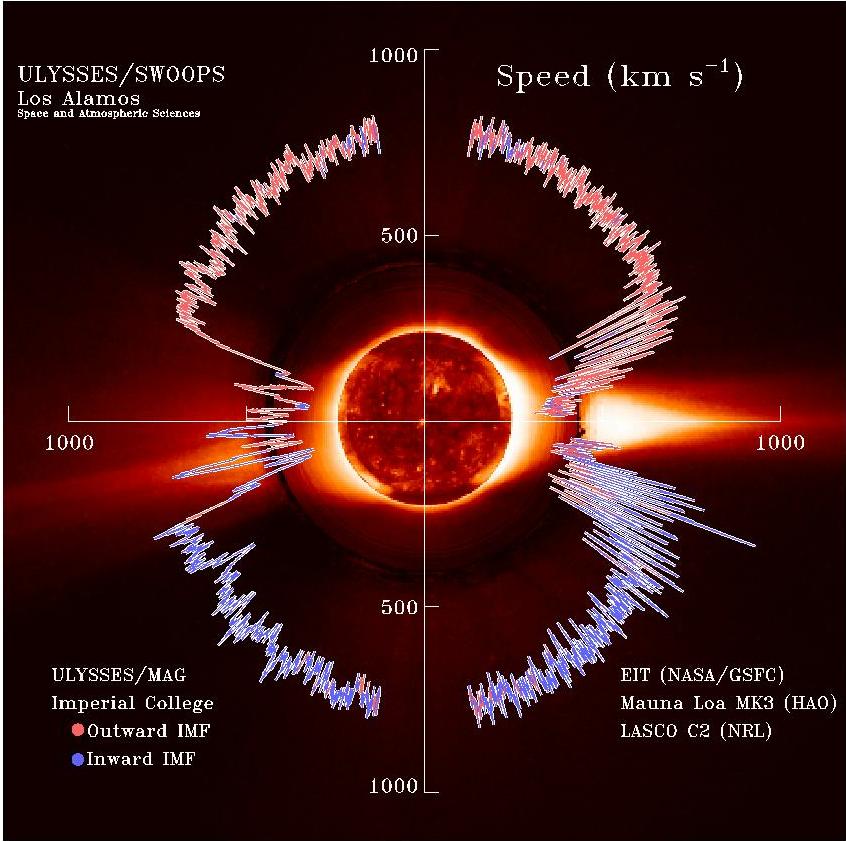
Solar Wind
The solar wind is a stream of charged particles released from the upper atmosphere of the Sun, called the corona. This plasma mostly consists of electrons, protons and alpha particles with kinetic energy between . The composition of the solar wind plasma also includes a mixture of materials found in the solar plasma: trace amounts of heavy ions and atomic nuclei such as C, N, O, Ne, Mg, Si, S, and Fe. There are also rarer traces of some other nuclei and isotopes such as P, Ti, Cr, 54Fe and 56Fe, and 58Ni, 60Ni, and 62Ni. Superposed with the solar-wind plasma is the interplanetary magnetic field. The solar wind varies in density, temperature and speed over time and over solar latitude and longitude. Its particles can escape the Sun's gravity because of their high energy resulting from the high temperature of the corona, which in turn is a result of the coronal magnetic field. The boundary separating the corona from the solar wind is called the Alfvén surface. At a distance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
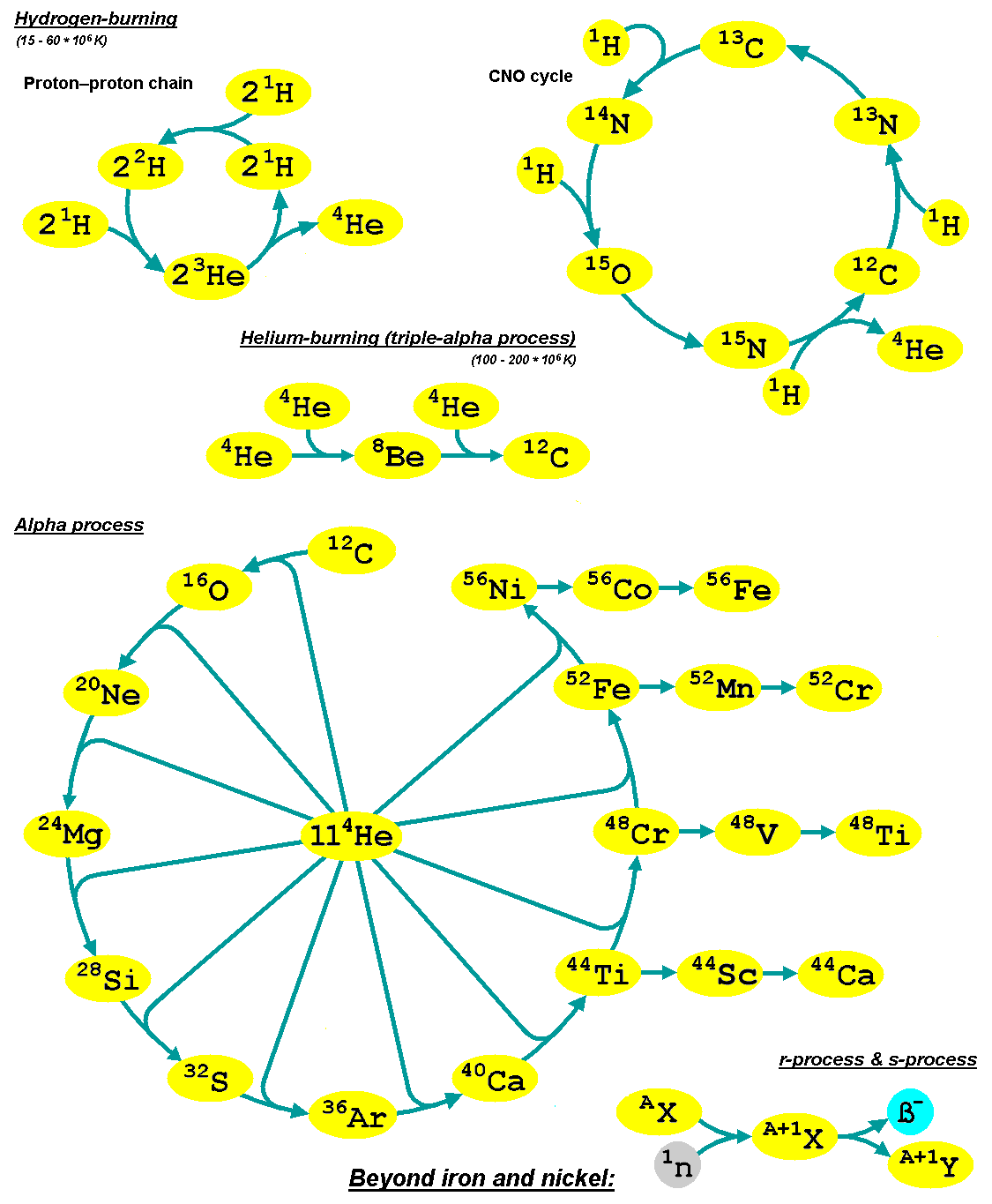
Alpha Process
The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements, the other being the triple-alpha process. The triple-alpha process consumes only helium, and produces carbon. After enough carbon has accumulated, further reactions below take place, listed below. Each step only consumes helium and the product of the previous reaction. :\begin \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \\ \ce& E=\mathsf \end The energy produced each the reaction, , is primarily in the gamma ray (), with a small amount taken by the byproduct element, as added momentum. It is a common misconception that the above sequence ends at \, _^\mathrm \, (or \, _^\mathrm \,, which is a decay product of \, _^\mathrm \,) because it is the most tightly bound nuclide - i.e., having the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stellar Nucleosynthesis
Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a predictive theory, it yields accurate estimates of the observed abundances of the elements. It explains why the observed abundances of elements change over time and why some elements and their isotopes are much more abundant than others. The theory was initially proposed by Fred Hoyle in 1946, who later refined it in 1954. Further advances were made, especially to nucleosynthesis by neutron capture of the elements heavier than iron, by Margaret and Geoffrey Burbidge, William Alfred Fowler and Hoyle in their famous 1957 B2FH paper, which became one of the most heavily cited papers in astrophysics history. Stars evolve because of changes in their composition (the abundance of their constituent elements) over their lifespans, first ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium-40
Potassium-40 (40K) is a radioactive isotope of potassium which has a long half-life of 1.25 billion years. It makes up about 0.012% (120 ppm) of the total amount of potassium found in nature. Potassium-40 undergoes three types of radioactive decay. In about 89.28% of events, it decays to calcium-40 (40Ca) with emission of a beta particle (β−, an electron) with a maximum energy of 1.31 MeV and an antineutrino. In about 10.72% of events, it decays to argon-40 (40Ar) by electron capture (EC), with the emission of a neutrino and then a 1.460 MeV gamma ray. The radioactive decay of this particular isotope explains the large abundance of argon (nearly 1%) in the Earth's atmosphere, as well as prevalence of 40Ar over other isotopes. Very rarely (0.001% of events), it decays to 40Ar by emitting a positron (β+) and a neutrino. Potassium–argon dating Potassium-40 is especially important in potassium–argon (K–Ar) dating. Argon is a gas that does not ordinarily combin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar System" and "solar system" structures in theinaming guidelines document. The name is commonly rendered in lower case ('solar system'), as, for example, in the ''Oxford English Dictionary'' an''Merriam-Webster's 11th Collegiate Dictionary''. is the gravitationally bound system of the Sun and the objects that orbit it. It formed 4.6 billion years ago from the gravitational collapse of a giant interstellar molecular cloud. The vast majority (99.86%) of the system's mass is in the Sun, with most of the remaining mass contained in the planet Jupiter. The four inner system planets— Mercury, Venus, Earth and Mars—are terrestrial planets, being composed primarily of rock and metal. The four giant planets of the outer system are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Number
The mass number (symbol ''A'', from the German word ''Atomgewicht'' tomic weight, also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approximately equal to the ''atomic'' (also known as ''isotopic'') mass of the atom expressed in atomic mass units. Since protons and neutrons are both baryons, the mass number ''A'' is identical with the baryon number ''B'' of the nucleus (and also of the whole atom or ion). The mass number is different for each isotope of a given chemical element, and the difference between the mass number and the atomic number ''Z'' gives the number of neutrons (''N'') in the nucleus: . The mass number is written either after the element name or as a superscript to the left of an element's symbol. For example, the most common isotope of carbon is carbon-12, or , which has 6 protons and 6 neutrons. The full isotope symbol would also have the atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Element
A synthetic element is one of 24 known chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, they are called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table: these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons into the nucleus of an element with an atomic number lower than 95. All synthetic elements are unstable, but they decay at widely varying rates: the half-lives of their longest-lived isotopes range from microseconds to millions of years. Five more elements that were created artificially are strictly speaking not ''synthetic'' because they were later found in nature in trace quantities: 43Tc, 61Pm, 85At, 93Np, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |