|
Soil Salinization
Soil salinity is the salt content in the soil; the process of increasing the salt content is known as salinization. Salts occur naturally within soils and water. Salination can be caused by natural processes such as mineral weathering or by the gradual withdrawal of an ocean. It can also come about through artificial processes such as irrigation and road salt. Natural occurrence Salts are a natural component in soils and water. The ions responsible for salination are: Na+, K+, Ca2+, Mg2+ and Cl−. Over long periods of time, as soil minerals weather and release salts, these salts are flushed or leached out of the soil by drainage water in areas with sufficient precipitation. In addition to mineral weathering, salts are also deposited via dust and precipitation. Salts may accumulate in dry regions, leading to naturally saline soils. This is the case, for example, in large parts of Australia. Human practices can increase the salinity of soils by the addition of salts in i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
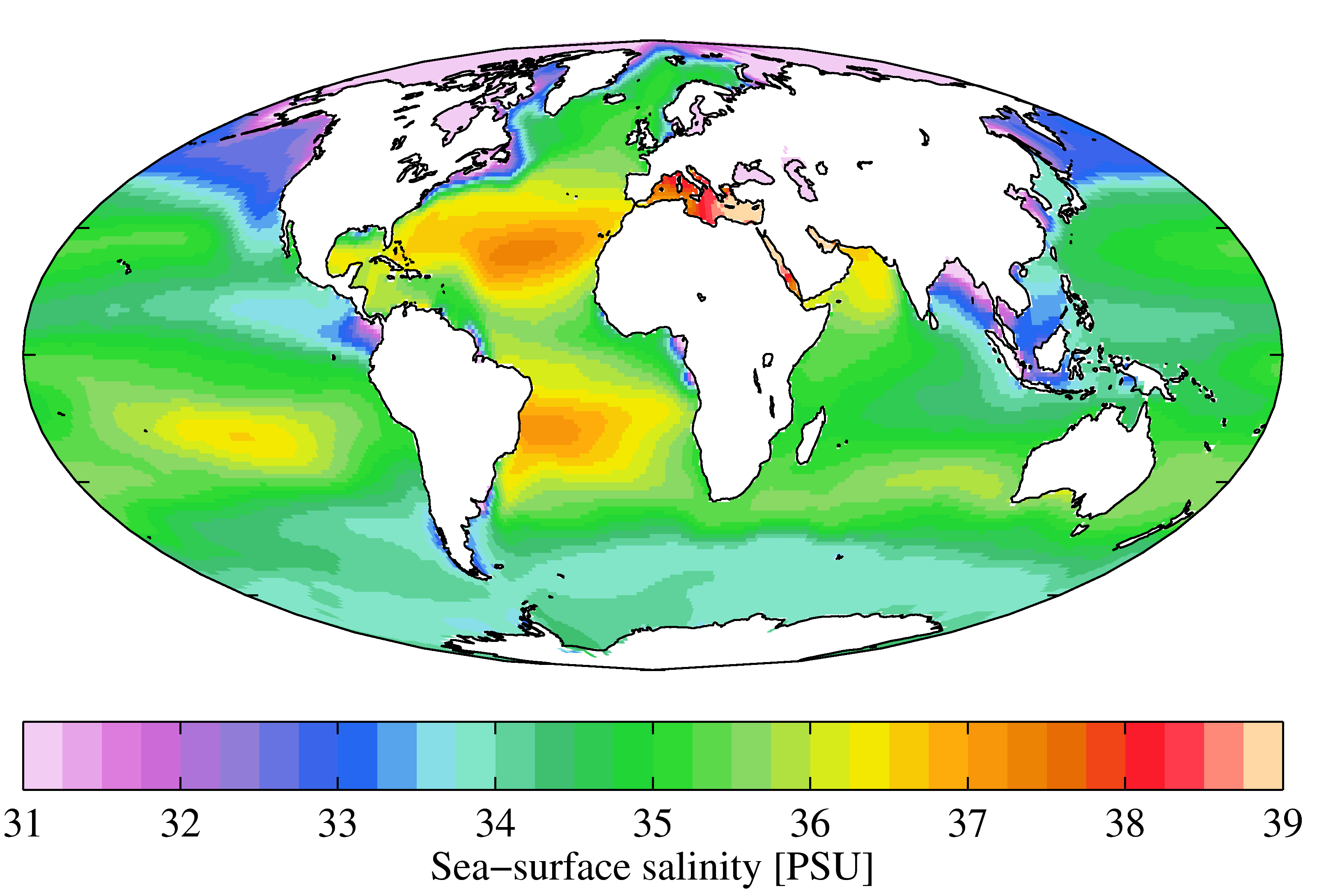
Salinity
Salinity () is the saltiness or amount of salt (chemistry), salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to ‰). Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it, and is a state function, thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water. A contour line of constant salinity is called an ''isohaline'', or sometimes ''isohale''. Definitions Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds like sodium chloride, magnesium sulfate, potassium nitrate, and sodium bicarbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ground Water
Groundwater is the water present beneath Earth's surface in rock and soil pore spaces and in the fractures of rock formations. About 30 percent of all readily available freshwater in the world is groundwater. A unit of rock or an unconsolidated deposit is called an aquifer when it can yield a usable quantity of water. The depth at which soil pore spaces or fractures and voids in rock become completely saturated with water is called the water table. Groundwater is recharged from the surface; it may discharge from the surface naturally at springs and seeps, and can form oases or wetlands. Groundwater is also often withdrawn for agricultural, municipal, and industrial use by constructing and operating extraction wells. The study of the distribution and movement of groundwater is hydrogeology, also called groundwater hydrology. Typically, groundwater is thought of as water flowing through shallow aquifers, but, in the technical sense, it can also contain soil moisture, permafro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Soil
Alkali, or Alkaline, soils are clay soils with high pH (greater than 8.5), a poor soil structure and a low infiltration capacity. Often they have a hard calcareous layer at 0.5 to 1 metre depth. Alkali soils owe their unfavorable physico-chemical properties mainly to the dominating presence of sodium carbonate, which causes the soil to swellManaging irrigation water quality, Oregon State University, USA Retrieved on 2012-10-04. and difficult to clarify/settle. They derive their name from the group of elements, to which |
Ion Toxicity
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywall. Alabaster, a fine-grained white or lightly tinted variety of gypsum, has been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on scratch hardness comparison. Etymology and history The word '' gypsum'' is derived from the Greek word (), "plaster". Because the quarries of the Montmartre district of Paris have long furnished burnt gypsum (calcined gypsum) used for various purposes, this dehydrated gypsum becam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Land Reclamation
Land reclamation, usually known as reclamation, and also known as land fill (not to be confused with a waste landfill), is the process of creating new land from oceans, seas, riverbeds or lake beds. The land reclaimed is known as reclamation ground or land fill. In some jurisdictions, including parts of the United States, the term "reclamation" can refer to returning disturbed lands to an improved state. In Alberta, Canada, for example, reclamation is defined by the provincial government as "The process of reconverting disturbed land to its former or other productive uses." In Oceania, it is frequently referred to as land rehabilitation. History One of the earliest large-scale projects was the Beemster Polder in the Netherlands, realized in 1612 adding of land. In Hong Kong the Praya Reclamation Scheme added of land in 1890 during the second phase of construction. It was one of the most ambitious projects ever taken during the Colonial Hong Kong era.Bard, Solomon. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhizosphere
The rhizosphere is the narrow region of soil or substrate that is directly influenced by root secretions and associated soil microorganisms known as the root microbiome. Soil pores in the rhizosphere can contain many bacteria and other microorganisms that feed on sloughed-off plant cells, termed ''rhizodeposition'', and the proteins and sugars released by roots, termed root exudates. This symbiosis leads to more complex interactions, influencing plant growth and competition for resources. Much of the nutrient cycling and disease suppression by antibiotics required by plants, occurs immediately adjacent to roots due to root exudates and metabolic products of symbiotic and pathogenic communities of microorganisms. The rhizosphere also provides space to produce allelochemicals to control neighbours and relatives. The ''rhizoplane'' refers to the root surface including its associated soil particles which closely interact with each other. The plant-soil feedback loop and other phy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm. Molybdenum does not occur naturally as a free metal on Earth; it is found only in various oxidation states in minerals. The free element, a silvery metal with a grey cast, has the sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys. Most molybdenum compounds have low solubility ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is a meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Infiltration
Infiltration is the process by which water on the ground surface enters the soil. It is commonly used in both hydrology and soil sciences. The infiltration capacity is defined as the maximum rate of infiltration. It is most often measured in meters per day but can also be measured in other units of distance over time if necessary. The infiltration capacity decreases as the soil moisture content of soils surface layers increases. If the precipitation rate exceeds the infiltration rate, runoff will usually occur unless there is some physical barrier. Infiltrometers, permeameters and rainfall simulators are all devices that can be used to measure infiltration rates. Infiltration is caused by multiple factors including; gravity, capillary forces, adsorption and osmosis. Many soil characteristics can also play a role in determining the rate at which infiltration occurs. Factors that affect infiltration Precipitation Precipitation can impact infiltration in many ways. The am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodic Soil
Soil salinity is the salt content in the soil; the process of increasing the salt content is known as salinization. Salts occur naturally within soils and water. Salination can be caused by natural processes such as mineral weathering or by the gradual withdrawal of an ocean. It can also come about through artificial processes such as irrigation and road salt. Natural occurrence Salts are a natural component in soils and water. The ions responsible for salination are: Na+, K+, Ca2+, Mg2+ and Cl−. Over long periods of time, as soil minerals weather and release salts, these salts are flushed or leached out of the soil by drainage water in areas with sufficient precipitation. In addition to mineral weathering, salts are also deposited via dust and precipitation. Salts may accumulate in dry regions, leading to naturally saline soils. This is the case, for example, in large parts of Australia. Human practices can increase the salinity of soils by the addition of salts in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Soil
Alkali, or Alkaline, soils are clay soils with high pH (greater than 8.5), a poor soil structure and a low infiltration capacity. Often they have a hard calcareous layer at 0.5 to 1 metre depth. Alkali soils owe their unfavorable physico-chemical properties mainly to the dominating presence of sodium carbonate, which causes the soil to swellManaging irrigation water quality, Oregon State University, USA Retrieved on 2012-10-04. and difficult to clarify/settle. They derive their name from the group of elements, to which sodium belon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |