|
Startup Neutron Source
A startup neutron source is a neutron source used for stable and reliable initiation of nuclear chain reaction in nuclear reactors, when they are loaded with fresh nuclear fuel, whose neutron flux from spontaneous fission is insufficient for a reliable startup, or after prolonged shutdown periods. Neutron sources ensure a constant minimal population of neutrons in the reactor core, sufficient for a smooth startup. Without them, the reactor could suffer fast power excursions during startup from state with too few self-generated neutrons (new core or after extended shutdown). The startup sources are typically inserted in regularly spaced positions inside the reactor core, in place of some of the fuel rods. The sources are important for safe reactor startup. The spontaneous fission and ambient radiation such as cosmic rays serve as weak neutron sources, but these are too weak for the reactor instrumentation to detect; relying on them could lead to a "blind" start, which is a pote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Neutron Source
A neutron source is any device that emits neutrons, irrespective of the mechanism used to produce the neutrons. Neutron sources are used in physics, engineering, medicine, nuclear weapons, petroleum exploration, biology, chemistry, and nuclear power. Neutron source variables include the energy of the neutrons emitted by the source, the rate of neutrons emitted by the source, the size of the source, the cost of owning and maintaining the source, and government regulations related to the source. Small devices Spontaneous fission Some isotopes undergo spontaneous fission (SF) with Neutron emission, emission of neutrons. The most common spontaneous fission source is the isotope californium-252. 252Cf and all other SF neutron sources are made by irradiating uranium or a Transuranium element, transuranic element in a nuclear reactor, where neutrons are absorbed in the starting material and its subsequent reaction products, transmuting the starting material into the SF isotope. 252Cf n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Radium
Radium is a chemical element; it has chemical symbol, symbol Ra and atomic number 88. It is the sixth element in alkaline earth metal, group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather than oxygen) upon exposure to air, forming a black surface layer of radium nitride (Ra3N2). All isotopes of radium are radioactive, the most stable isotope being radium-226 with a half-life of 1,600 years. When radium decays, it emits ionizing radiation as a by-product, which can excite fluorescent chemicals and cause radioluminescence. For this property, it was widely used in Self-luminous paint, self-luminous paints following its discovery. Of the Radionuclide, radioactive elements that occur in quantity, radium is considered particularly Toxicity, toxic, and it is Carcinogen, carcinogenic due to the radioactivity of both it and its immediate decay product radon as well as its tendency to B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chicago Pile-1
Chicago Pile-1 (CP-1) was the first artificial nuclear reactor. On 2 December 1942, the first human-made self-sustaining nuclear chain reaction was initiated in CP-1 during an experiment led by Enrico Fermi. The secret development of the reactor was the first major technical achievement for the Manhattan Project, the Allies of World War II, Allied effort to create nuclear weapons during World War II. Developed by the Metallurgical Laboratory at the University of Chicago, CP-1 was built under the west viewing stands of the original Stagg Field. Although the project's civilian and military leaders had misgivings about the possibility of a disastrous runaway reaction, they trusted Fermi's safety calculations and decided they could carry out the experiment in a densely populated area. Fermi described the reactor as "a crude pile of black bricks and wooden timbers". After a series of attempts, the successful reactor was assembled in November 1942 by a team of about 30 that, in addi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have properties that differ from those of the pure elements from which they are made. The vast majority of metals used for commercial purposes are alloyed to improve their properties or behavior, such as increased strength, hardness or corrosion resistance. Metals may also be alloyed to reduce their overall cost, for instance alloys of gold and Copper(II) sulfate, copper. A typical example of an alloy is SAE 304 stainless steel, 304 grade stainless steel which is commonly used for kitchen utensils, pans, knives and forks. Sometime also known as 18/8, it as an alloy consisting broadly of 74% iron, 18% chromium and 8% nickel. The chromium and nickel alloying elements add strength and hardness to the majority iron element, but their main function is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Stainless Steel
Stainless steel, also known as inox, corrosion-resistant steel (CRES), or rustless steel, is an iron-based alloy that contains chromium, making it resistant to rust and corrosion. Stainless steel's resistance to corrosion comes from its chromium content of 11% or more, which forms a Passivation (chemistry), passive film that protects the material and can self-healing material, self-heal when exposed to oxygen. It can be further alloyed with elements like molybdenum, carbon, nickel and nitrogen to enhance specific properties for various applications. The alloy's properties, such as luster and resistance to corrosion, are useful in many applications. Stainless steel can be rolled into Sheet metal, sheets, plates, bars, wire, and tubing. These can be used in cookware, cutlery, surgical instruments, major appliances, vehicles, construction material in large buildings, industrial equipment (e.g., in paper mills, chemical plants, water treatment), and storage tanks and tankers for ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
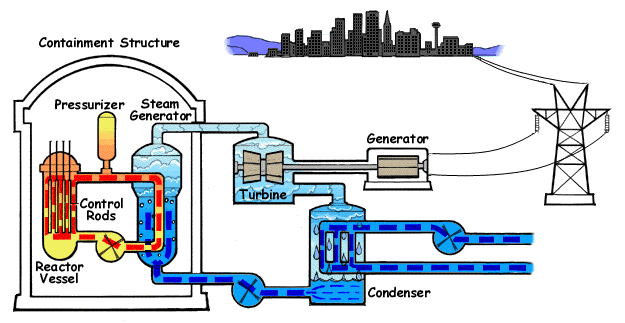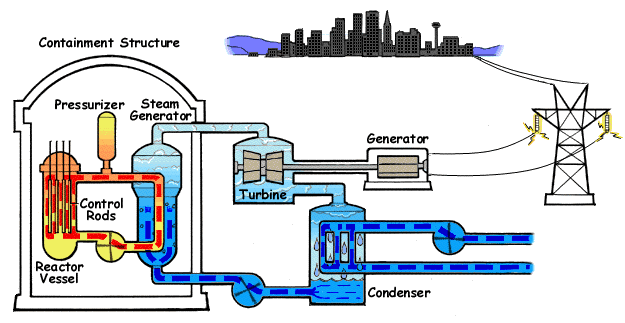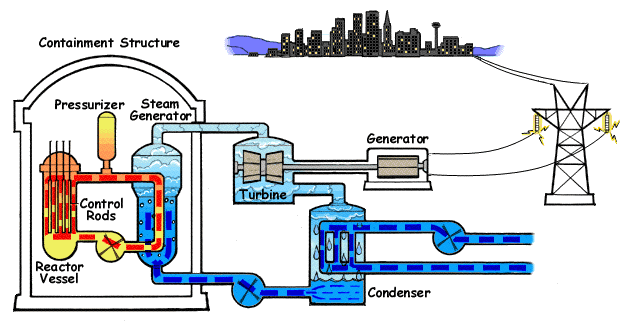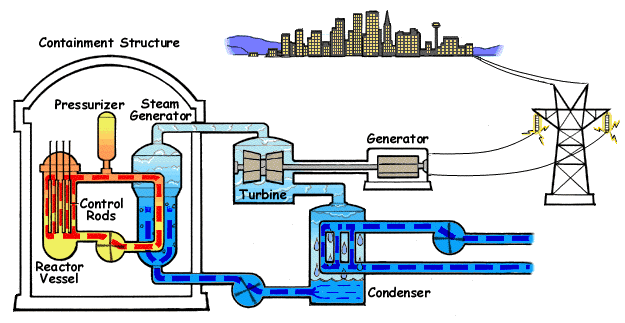
Pressurized Water Reactor
A pressurized water reactor (PWR) is a type of light-water nuclear reactor. PWRs constitute the large majority of the world's nuclear power plants (with notable exceptions being the UK, Japan, India and Canada). In a PWR, water is used both as a neutron moderator and as coolant fluid for the reactor core. In the core, water is heated by the energy released by the fission of atoms contained in the fuel. Using very high pressure (around 155 bar: 2250 psi) ensures that the water stays in a liquid state. The heated water then flows to a steam generator, where it transfers its thermal energy to the water of a secondary cycle kept at a lower pressure which allows it to vaporize. The resulting steam then drives steam turbines linked to an electric generator. A boiling water reactor (BWR) by contrast does not maintain such a high pressure in the primary cycle and the water thus vaporizes inside of the reactor pressure vessel (RPV) before being sent to the turbine. Most PWR designs ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Beryllium-9
Beryllium (4Be) has 11 known isotopes and 3 known isomers, but only one of these isotopes () is stable and a primordial nuclide. As such, beryllium is considered a monoisotopic element. It is also a mononuclidic element, because its other isotopes have such short half-lives that none are primordial and their abundance is very low (standard atomic weight is ). Beryllium is unique as being the only monoisotopic element with both an even number of proton A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...s and an odd number of neutrons. There are 25 other monoisotopic elements but all have odd atomic numbers, and even numbers of neutrons. Of the 10 radionuclide, radioisotopes of beryllium, the most stable are with a half-life of million years and with a half-life of . All other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Photoneutron
Photodisintegration (also called phototransmutation, or a photonuclear reaction) is a nuclear process in which an atomic nucleus absorbs a high-energy gamma ray, enters an excited state, and immediately decays by emitting a subatomic particle. The incoming gamma ray effectively knocks one or more neutrons, protons, or an alpha particle out of the nucleus. The reactions are called (γ,n), (γ,p), and (γ,α), respectively. Photodisintegration is endothermic (energy absorbing) for atomic nuclei lighter than iron and sometimes exothermic (energy releasing) for atomic nuclei heavier than iron. Photodisintegration is responsible for the nucleosynthesis of at least some heavy, proton-rich elements via the p-process in supernovae of type Ib, Ic, or II. This causes the iron to further fuse into the heavier elements. Photodisintegration of deuterium A photon carrying 2.22 MeV or more energy can photodisintegrate an atom of deuterium: : James Chadwick and Maurice Goldhaber used this re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Beryllium
Beryllium is a chemical element; it has Symbol (chemistry), symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form minerals. Gemstones high in beryllium include beryl (Aquamarine (gemstone), aquamarine, emerald, red beryl) and chrysoberyl. It is a Abundance of the chemical elements#Universe, relatively rare element in the universe, usually occurring as a product of the spallation of larger atomic nuclei that have collided with cosmic rays. Within the cores of stars, beryllium is depleted as it is fused into heavier elements. Beryllium constitutes about 0.0004 percent by mass of Earth's crust. The world's annual beryllium production of 220 tons is usually manufactured by extraction from the mineral beryl, a difficult process because beryllium bonds strongly to oxygen. In structural applications, the combination of high flexural ri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name Kohl (cosmetics), kohl. The earliest known description of this metalloid in the West was written in 1540 by Vannoccio Biringuccio. China is the largest producer of antimony and its compounds, with most production coming from the Xikuangshan Mine in Hunan. The industrial methods for refining antimony from stibnite are Roasting (metallurgy), roasting followed by carbothermic reaction, reduction with carbon, or direct reduction of stibnite with iron. The most common applications for metallic antimony are in alloys with lead and tin, which have improved properties for solders, Bullet, bullets, and plain bearings. It improves the rigidity of lead-alloy pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Neutron Activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emitting gamma rays, or particles such as beta particles, alpha particles, fission products, and neutrons (in nuclear fission). Thus, the process of neutron capture, even after any intermediate decay, often results in the formation of an unstable activation product. Such radioactive nuclei can exhibit half-lives ranging from small fractions of a second to many years. Neutron activation is the only common way that a stable material can be induced into becoming intrinsically radioactive. All naturally occurring materials, including air, water, and soil, can be induced (activated) by neutron capture into some amount of radioactivity in varying degrees, as a result of the production of neutron-rich radioisotopes. Some atoms require more th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Fast Neutron
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is then adapted to the Maxwell distribution known for thermal motion. Qualitatively, the higher the temperature, the higher the kinetic energy of the free neutrons. The momentum and wavelength of the neutron are related through the de Broglie relation. The long wavelength of slow neutrons allows for the large cross section. Neutron energy distribution ranges The precise boundaries of neutron energy ranges are not well defined, and differ between sources, but some common names and limits are given in the following table. The following is a detailed classification: Thermal A thermal neutron is a free neutron with a kinetic energy of about 0.025 eV (about 4.0×10−21 J or 2. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |