|
Solid-state Silicon Battery
A solid-state silicon battery or silicon-anode all-solid-state battery is a type of rechargeable lithium-ion battery consisting of a solid electrolyte, solid cathode, and silicon-based solid anode. In solid-state silicon batteries, lithium ions travel through a solid electrolyte from a positive cathode to a negative silicon anode. While silicon anodes for lithium-ion batteries have been studied, they were largely dismissed as infeasible due to general incompatibility with liquid electrolytes. Developments in 2021 showed that solid-state silicon lithium-ion batteries are possible, and offer many of the hypothesized benefits. Solid electrolytes more easily interface with the anode. These batteries are different from other solid-state batteries due to their use of silicon instead of less energy-dense materials. Silicon is difficult to work with because it expands over 300% during lithiation (also known as lithium intercalation). This contributes to the other major difficulty: lithium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium-ion Battery
A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. Li-ion batteries are characterized by higher specific energy, energy density, and energy efficiency and a longer cycle life and calendar life than other types of rechargeable batteries. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991; over the following 30 years, their volumetric energy density increased threefold while their cost dropped tenfold. In late 2024 global demand passed per year, while production capacity was more than twice that. The invention and commercialization of Li-ion batteries has had a large impact on technology, as recognized by the 2019 Nobel Prize in Chemistry. Li-ion batteries have enabled portable consumer electronics, laptop computers, cellular phones, and electric cars. Li-ion batteries also see signifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast-ion Conductor
In materials science, fast ion conductors are solid conductors with highly mobile ions. These materials are important in the area of solid state ionics, and are also known as solid electrolytes and superionic conductors. These materials are useful in batteries and various sensors. Fast ion conductors are used primarily in solid oxide fuel cells. As solid electrolytes they allow the movement of ions without the need for a liquid or soft membrane separating the electrodes. The phenomenon relies on the hopping of ions through an otherwise rigid crystal structure. Mechanism Fast ion conductors are intermediate in nature between crystalline solids which possess a regular structure with immobile ions, and liquid electrolytes which have no regular structure and fully mobile ions. Solid electrolytes find use in all solid-state supercapacitors, batteries, and fuel cells, and in various kinds of chemical sensors. Classification In solid electrolytes (glasses or crystals), the ionic conduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
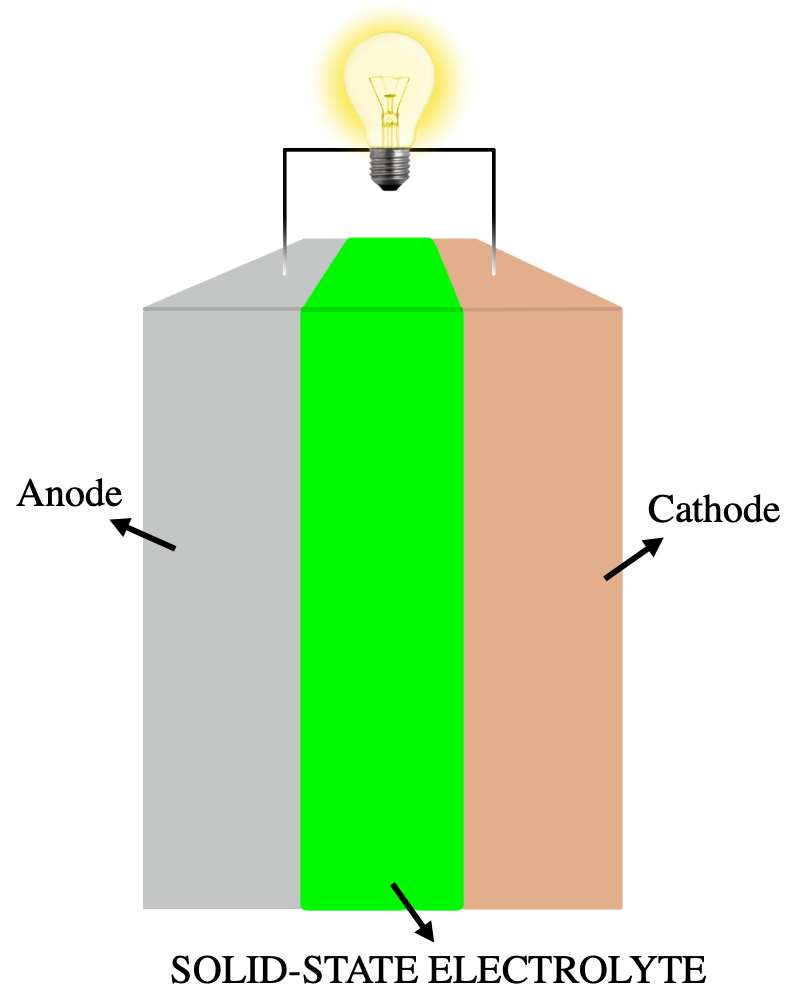
Cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional current describes the direction in which positive charges move. Electrons, which are the carriers of current in most electrical systems, have a negative electrical charge, so the movement of electrons is ''opposite'' to that of the conventional current flow: this means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the device. A common mnemonic is ACID, for "anode current into device". The direction of conventional current (the flow of positive charges) in a circuit is opposite to the direction of electron flow, so (negatively charged) electrons flow from the anode of a galvanic cell, into an outside or external circuit connected to the cell. For example, the end of a household battery marked with a "+" is the cathode (while discharging). In both a galvanic cell and an electrolytic cell, the anode is the electrode at which the oxidation reaction occurs. In a galvanic cell the anode is the wire or plate having excess negative charge as a result of the oxidation reaction. In an electrolytic cell, the anode is the wire or plate upon which excess positive charge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solvent like water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also functi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid-state Battery
A solid-state battery (SSB) is an electrical battery that uses a solid electrolyte (''solectro'') to conduct ions between the electrodes, instead of the liquid or gel polymer electrolytes found in conventional batteries. Solid-state batteries theoretically offer much higher energy density than the typical lithium-ion or lithium polymer batteries. While solid electrolytes were first discovered in the 19th century, several problems prevented widespread application. Developments in the late 20th and early 21st century generated renewed interest in the technology, especially in the context of electric vehicles. Solid-state batteries can use metallic lithium for the anode and oxides or sulfides for the cathode, increasing energy density. The solid electrolyte acts as an ideal separator that allows only lithium ions to pass through. For that reason, solid-state batteries can potentially solve many problems of currently used liquid electrolyte Li-ion batteries, such as flammabil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intercalation (chemistry)
Intercalation is the reversible inclusion or insertion of a molecule (or ion) into layered materials with layered structures. Examples are found in graphite and transition metal dichalcogenides. : Examples Graphite One famous intercalation host is graphite, which intercalates potassium as a guest. Intercalation expands the van der Waals gap between sheets, which requires energy. Usually this energy is supplied by charge transfer between the guest and the host solid, i.e., redox. Two potassium graphite compounds are KC8 and KC24. Carbon fluorides (e.g., (CF)x and (C4F)) are prepared by reaction of fluorine with graphitic carbon. The color is greyish, white, or yellow. The bond between the carbon and fluorine atoms is covalent, thus fluorine is not intercalated. Such materials have been considered as a cathode in various lithium batteries. Treating graphite with strong acids in the presence of oxidizing agents causes the graphite to oxidise. Graphite bisulfate, 24sup>+ SO4sup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dendrite (crystal)
A crystal dendrite is a crystal that develops with a typical multi-branching form, resembling a fractal. The name comes from the Ancient Greek word (), which means "tree", since the crystal's structure resembles that of a tree. These crystals can be synthesised by using a supercooled pure liquid, however they are also quite common in nature. The most common crystals in nature exhibit dendritic growth are snowflakes and frost on windows, but many minerals and metals can also be found in dendritic structures. History Maximum velocity principle The first dendritic patterns were discovered in palaeontology and are often mistaken for fossils because of their appearance. The first theory for the creation of these patterns was published by Nash and Glicksman in 1974, they used a very mathematical method and derived a non-linear integro-differential equation for a classical needle growth. However they only found an inaccurate numerical solution close to the tip of the needle a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of California, San Diego
The University of California, San Diego (UC San Diego in communications material, formerly and colloquially UCSD) is a public university, public Land-grant university, land-grant research university in San Diego, California, United States. Established in 1960 near the pre-existing Scripps Institution of Oceanography in La Jolla, UC San Diego is the southernmost of the ten campuses of the University of California. It offers over 200 undergraduate and graduate degree programs, enrolling 33,096 undergraduate and 9,872 graduate students, with the second largest student housing capacity in the nation. The university occupies near the Pacific coast. UC San Diego consists of 12 undergraduate, graduate, and professional schools as well as 8 undergraduate residential colleges. The university operates 19 organized research units as well as 8 School of Medicine research units, 6 research centers at Scripps Institution of Oceanography, and 2 multi-campus initiatives. UC San Diego is als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LG Corporation
LG Corporation (or LG Group), formerly known as Lucky-Goldstar, is a South Korean multinational conglomerate founded by Koo In-hwoi in 1947 and managed by successive generations of his family. It is the fourth-largest company in South Korea. Its headquarters are in the LG Twin Towers building in Yeouido-dong, Yeongdeungpo District, Seoul. LG makes electronics, chemicals, household appliances, and telecommunications products and operates subsidiaries such as LG Electronics, Zenith, LG Display, LG Uplus, LG Innotek, LG Chem, LG Energy Solution and LG AI Research in over 80 countries. History LG Corporation was established as Lak Hui Chemical Industrial Corp. in 1947 by Koo In-hwoi. Its first product was "Lucky Cream", the first Korean make-up cream. In 1952, Lak Hui () (pronounced "Lucky"; now LG Chem) became the first South Korean company to enter the plastics industry. As the company expanded its plastics business, it established GoldStar Co. Ltd. (now LG Electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Energy
Specific energy or massic energy is energy per unit mass. It is also sometimes called gravimetric energy density, which is not to be confused with energy density, which is defined as energy per unit volume. It is used to quantify, for example, stored heat and other thermodynamic properties of substances such as specific internal energy, specific enthalpy, specific Gibbs free energy, and specific Helmholtz free energy. It may also be used for the kinetic energy or potential energy of a body. Specific energy is an intensive property, whereas energy and mass are extensive properties. The SI unit for specific energy is the joule per kilogram (J/kg). Other units still in use worldwide in some contexts are the kilocalorie per gram (Cal/g or kcal/g), mostly in food-related topics, and watt-hours per kilogram (W⋅h/kg) in the field of batteries. In some countries the Imperial unit BTU per pound (Btu/lb) is used in some engineering and applied technical fields. Kenneth E. Hese ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid-state Electrolyte
A solid-state electrolyte (SSE) is a solid Ionic conductivity (solid state), ionic conductor and electron-insulating electrolyte, material and it is the characteristic component of the solid-state battery. It is useful for applications in electrical energy storage in substitution of the liquid electrolytes found in particular in the lithium-ion battery. Their main advantages are their absolute safety, no issues of leakages of toxic organic solvents, low flammability, non-volatility, mechanical and thermal stability, easy processability, low self-discharge, higher achievable power density and cyclability. This makes possible, for example, the use of a lithium metal anode in a practical device, without the intrinsic limitations of a liquid electrolyte thanks to the property of lithium Dendrite (crystal), dendrite suppression in the presence of a solid-state electrolyte membrane. The use of a high-capacity and low reduction potential anode, like lithium with a specific capacity of 386 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |