|
Salicylaldehyde
Salicylic aldehyde (2-hydroxybenzaldehyde) is an organic compound with the formula . Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration. Salicylaldehyde is a precursor to coumarin and a variety of chelating agents. Production Salicylaldehyde is produced by condensation of phenol with formaldehyde to give hydroxybenzyl alcohol, which is oxidized to the aldehyde. Salicylaldehydes in general are prepared by ortho-selective formylation reactions from the corresponding phenol, for instance by the Duff reaction, Reimer–Tiemann reaction, or by treatment with paraformaldehyde in the presence of magnesium chloride and a base. : Natural occurrences Salicylaldehyde is a characteristic aroma component of buckwheat. Salicylaldehyde also occurs in the larval defensive secretions of several leaf beetle species that belong the subtribe Chryso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rap–Stoermer Condensation
Salicylic aldehyde (2-hydroxybenzaldehyde) is an organic compound with the formula . Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration. Salicylaldehyde is a precursor to coumarin and a variety of chelating agents. Production Salicylaldehyde is produced by condensation of phenol with formaldehyde to give hydroxybenzyl alcohol, which is oxidized to the aldehyde. Salicylaldehydes in general are prepared by ortho-selective formylation reactions from the corresponding phenol, for instance by the Duff reaction, Reimer–Tiemann reaction, or by treatment with paraformaldehyde in the presence of magnesium chloride and a base. : Natural occurrences Salicylaldehyde is a characteristic aroma component of buckwheat. Salicylaldehyde also occurs in the larval defensive secretions of several leaf beetle species that belong the subtribe Chrysomelina.Pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buckwheat
Buckwheat (''Fagopyrum esculentum'') or common buckwheat is a flowering plant in the knotweed family Polygonaceae cultivated for its grain-like seeds and as a cover crop. Buckwheat originated around the 6th millennium BCE in the region of what is now Yunnan, Yunnan Province in southwestern China. The name "buckwheat" is used for several other species, such as ''Fagopyrum tataricum'', a domesticated food plant raised in Asia. Despite its name, buckwheat is not closely related to wheat. Buckwheat is not a cereal, nor is it a member of the Poaceae, grass family. It is related to sorrel, Polygonum, knotweed, and rhubarb. Buckwheat is considered a pseudocereal because the high starch content of the seeds enables buckwheat to be cooked and consumed like a cereal. Etymology The name "buckwheat" or "beech wheat" comes from its tetrahedral seeds, which resemble the much larger seeds of the beech nut from the beech, beech tree, and the fact that it is used like wheat. The word may be a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salicylaldoxime
Salicylaldoxime is an organic compound described by the formula C6H4CH=NOH-2-OH. It is the oxime of salicylaldehyde. This crystalline, colorless solid is a chelator and sometimes used in the analysis of samples containing transition metal ions, with which it often forms brightly coloured coordination complexes. Reactions Salicylaldoxime is the conjugate acid of a bidentate ligand: :2 C6H4CH=NOH-2-OH + Cu2+ → Cu(C6H4CH=NOH-2-O)2 + 2 H+ In highly acidic media, the ligand protonates, and the metal aquo complex and aldoxime are liberated. In this way the ligand is used as a recyclable extractant. It typically forms charge-neutral complexes with divalent metal ions. Analytical chemistry In the era when metals were analysed by spectrophotometry, many chelating ligands were developed that selectively formed brightly coloured complexes with particular metal ions. This methodology has been eclipsed with the introduction of inductively coupled plasma methodology. Salicyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-hydroxybenzaldehyde
3-Hydroxybenzaldehyde is an organic compound with the formula . It is a colorless solid although most samples appear tan. Two other isomers of hydroxybenzaldehyde exist. Preparation It has been prepared from 3-nitrobenzaldehyde in a sequence of nitro group reduction, diazotization of the amine, and hydrolysis. 3-hydroxybenzyl-alcohol dehydrogenase is an NADP-dependent enzyme that produces 3-hydroxybenzaldehyde from 3-hydroxybenzyl alcohol. Biomedical properties 3-Hydroxybenzaldehyde exhibits vasculoprotective effects by lowering vascular smooth muscle cell proliferation and endothelial cells inflammation. 3-Hydroxybenzaldehyde is used in the synthesis of monastrol. See also * Salicylaldehyde Salicylic aldehyde (2-hydroxybenzaldehyde) is an organic compound with the formula . Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almo ... (2-hydroxybenzaldehyde) * 4-Hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perkin Synthesis
The Perkin reaction is an organic reaction developed by English chemist William Henry Perkin in 1868 that is used to make cinnamic acids. It gives an α,β-unsaturated aromatic acid or α-substituted β-aryl acrylic acid by the aldol condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid. The alkali salt acts as a base catalyst, and other bases can be used instead. Several reviews have been written. Reaction mechanism Clear from the reaction mechanism, the anhydride of aliphatic acid must contain at least 2 α-H for the reaction to occur. The above mechanism is not universally accepted, as several other versions exist, including decarboxylation without acetic group transfer.Bansal, Raj K. (1998) ''Organic Reaction Mechanisms'', Tata McGraw Hill, 3rd Edition , pp. 199–201, . Applications * Salicylaldehyde converted to coumarin using acetic anhydride with acetate as base. * cinnamic acid is prepared from benzaldehyde, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaf Beetle
The beetle family Chrysomelidae, commonly known as leaf beetles, includes over 37,000 (and probably at least 50,000) species in more than 2,500 genera, making it one of the largest and most commonly encountered of all beetle families. Numerous subfamilies are recognized, but the precise taxonomy and systematics are likely to change with ongoing research. Leaf beetles are partially recognizable by their tarsal formula, which appears to be 4-4-4, but is actually 5-5-5 as the fourth tarsal segment is very small and hidden by the third. As with many taxa, no single character defines Chrysomelidae; instead, the family is delineated by a set of characters. Some lineages are only distinguished with difficulty from longhorn beetles (family Cerambycidae), namely by the antennae not arising from frontal tubercles. Members of former chrysomelid subfamilies ( Orsodacnidae and Megalopodidae) are also difficult to differentiate from true chrysomelids. Adult and larval leaf beetles feed o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
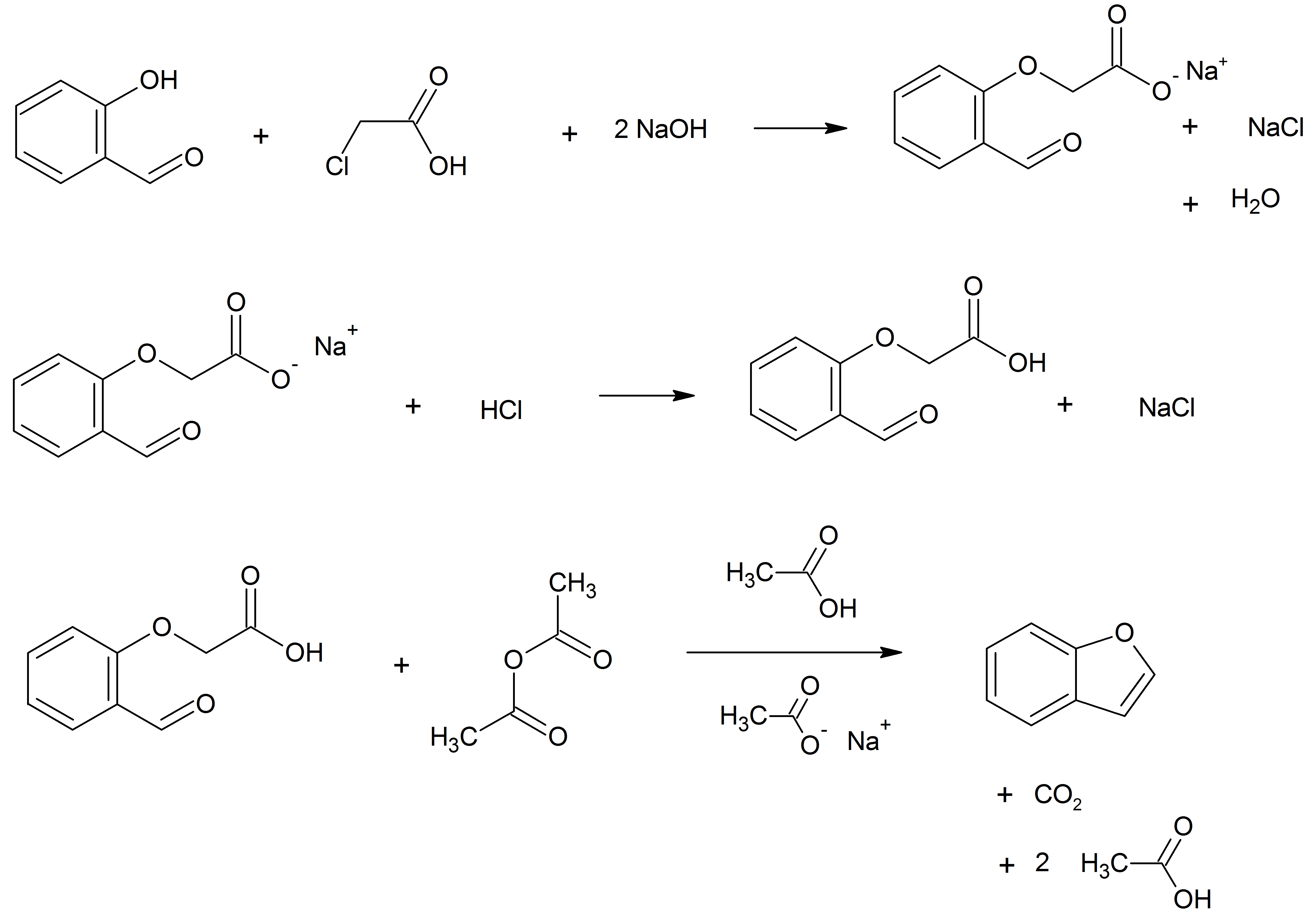
Benzofuran
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus (parent compound) of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethyl phenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. * Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : * Cycloisomerization of alkyne ortho-substituted phenols: : Related compounds * Substituted benzofurans * Dibenzofuran, an analog w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroacetic Acid
Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula . This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds are dichloroacetic acid and trichloroacetic acid. Production Chloroacetic acid was first prepared (in impure form) by the French chemist Félix LeBlanc (1813–1886) in 1843 by chlorinating acetic acid in the presence of sunlight, and in 1857 (in pure form) by the German chemist Reinhold Hoffmann (1831–1919) by refluxing glacial acetic acid in the presence of chlorine and sunlight, and then by the French chemist Charles Adolphe Wurtz by hydrolysis of chloroacetyl chloride (), also in 1857. Chloroacetic acid is prepared industrially by two routes. The predominant method involves chlorination of acetic acid, with acetic anhydride as a catalyst: : This route suffers from the production of dichloroacetic acid and trichloroacetic acid as i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Williamson Ether Synthesis
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. This reaction is important in the history of organic chemistry because it helped prove the structure of ethers. The general reaction mechanism is as follows: An example is the reaction of sodium ethoxide with chloroethane to form diethyl ether and sodium chloride: : Mechanism The Williamson ether reaction follows an SN2 (bimolecular nucleophilic substitution) mechanism. In an SN2 reaction mechanism there is a backside attack of an electrophile by a nucleophile and it occurs in a concerted mechanism (happens all at once). In order for the SN2 reaction to take place there must be a good leaving group which is strongly electronegative, commonly a halide. In the Williamson ether reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dakin Reaction
The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an '' ortho''- or ''para''-hydroxylated phenyl aldehyde ( 2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced. The Dakin oxidation, which is closely related to the Baeyer–Villiger oxidation, is not to be confused with the Dakin–West reaction, though both are named after Henry Drysdale Dakin. Reaction mechanism The Dakin oxidation starts with (1) nucleophilic addition of a hydroperoxide ion to the carbonyl carbon, forming a (2) tetrahedral intermediate. The intermediate collapses, causing ,2aryl migration, hydroxide elimination, and formation of a (3) phenyl ester. The phenyl ester is subsequently hydrolyzed: nucleophilic addition of hydroxide ion from solution to the ester carbonyl carbon forms a (4) second tetrahedral intermediate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is an organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances. Small amounts of catechol occur in fruits and vegetables. Isolation and synthesis Catechol was first isolated in 1839 by Edgar Hugo Emil Reinsch (1809–1884) by distilling it from the solid tannic preparation catechin, which is the residuum of catechu, the boiled or concentrated juice of ''Mimosa catechu'' ('' Acacia catechu''). Upon heating catechin above its decomposition point, a substance that Reinsch first named ''Brenz-Katechusäure'' (burned catechu acid) sublimated as a white efflo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |