|
Radurization
Food irradiation is the process of exposing food and food packaging to ionizing radiation, such as from gamma rays, x-rays, or electron beams. Food irradiation improves food safety and extends product shelf life (preservation) by effectively destroying organisms responsible for spoilage and foodborne illness, inhibits sprouting or ripening, and is a means of controlling insects and invasive pests. In the US, consumer perception of foods treated with irradiation is more negative than those processed by other means. The Food and Drug Administration, U.S. Food and Drug Administration (FDA), the World Health Organization (WHO), the Centers for Disease Control and Prevention (CDC), and United States Department of Agriculture, U.S. Department of Agriculture (USDA) have performed studies that confirm irradiation to be safe.World Health Organization. Safety and Nutritional Adequacy of Irradiated Food. Geneva, Switzerland: World Health Organization; 1994 In order for a food to be irrad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radura International
The Radura is the international symbol indicating a food irradiation, food product has been irradiated. The Radura is usually green and resembles a plant in circle. The top half of the circle is dashed. Graphical details and colours vary between countries. Meaning of the word "Radura" The word "Radura" is derived from radurization, in itself a portmanteau combining the initial letters of the word "radiation" with the stem of "durus", the Latin word for hard, lasting.D.A.E. Ehlermann, The Radura terminology and food irradiation, Food Control 20(2009), 526-528, History The inventors of the symbol Radura - knowing this proposal for a new terminology - came from the former Pilot Plant for Food Irradiation, Wageningen, Netherlands, which was the nucleus for the later Gammaster today known as Isotron. The director at the time, R.M. Ulmann, introduced this symbol to the international community. Ulmann in his lecture also provided the interpretation of this symbol: denoting food - ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reproduction
Reproduction (or procreation or breeding) is the biological process by which new individual organisms – "offspring" – are produced from their "parent" or parents. Reproduction is a fundamental feature of all known life; each individual organism exists as the result of reproduction. There are two forms of reproduction: asexual and sexual. In asexual reproduction, an organism can reproduce without the involvement of another organism. Asexual reproduction is not limited to single-celled organisms. The cloning of an organism is a form of asexual reproduction. By asexual reproduction, an organism creates a genetically similar or identical copy of itself. The evolution of sexual reproduction is a major puzzle for biologists. The two-fold cost of sexual reproduction is that only 50% of organisms reproduce and organisms only pass on 50% of their genes. John Maynard Smith ''The Evolution of Sex'' 1978. Sexual reproduction typically requires the sexual interaction of two speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Division
Cell division is the process by which a parent cell divides into two daughter cells. Cell division usually occurs as part of a larger cell cycle in which the cell grows and replicates its chromosome(s) before dividing. In eukaryotes, there are two distinct types of cell division: a vegetative division ( mitosis), producing daughter cells genetically identical to the parent cell, and a cell division that produces haploid gametes for sexual reproduction (meiosis), reducing the number of chromosomes from two of each type in the diploid parent cell to one of each type in the daughter cells. In cell biology, mitosis ( /maɪˈtoʊsɪs/) is a part of the cell cycle, in which, replicated chromosomes are separated into two new nuclei. Cell division gives rise to genetically identical cells in which the total number of chromosomes is maintained. In general, mitosis (division of the nucleus) is preceded by the S stage of interphase (during which the DNA replication occurs) and is oft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Changes
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., without breaking chemical bonds. Chemical substances can be simple substances (substances consisting of a single chemical element), chemical compounds, or alloys. Chemical substances are often called 'pure' to set them apart from mixtures. A common example of a chemical substance is pure water; it has the same properties and the same ratio of hydrogen to oxygen whether it is isolated from a river or made in a laboratory. Other chemical substances commonly encountered in pure form are diamond (carbon), gold, table salt ( sodium chloride) and refined sugar ( sucrose). However, in practice, no substance is entirely pure, and chemical purity is specified according to the intended use of the chemical. Chemical substances exist as solids, liqu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvated Electron
A solvated electron is a free electron in (solvated in) a solution, and is the smallest possible anion. Solvated electrons occur widely. Often, discussions of solvated electrons focus on their solutions in ammonia, which are stable for days, but solvated electrons also occur in water and other solvents in fact, in any solvent that mediates outer-sphere electron transfer. The solvated electron is responsible for a great deal of radiation chemistry. Ammonia solutions Liquid ammonia will dissolve all of the alkali metals and other electropositive metals such as Ca, Sr, Ba, Eu, and Yb (also Mg using an electrolytic process), giving characteristic blue solutions. For alkali metals in liquid ammonia, the solution is blue when dilute and copper-colored when more concentrated (> 3 molar). These solutions conduct electricity. The blue colour of the solution is due to ammoniated electrons, which absorb energy in the visible region of light. The diffusivity of the solvated electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
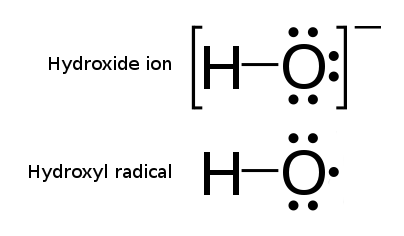
Hydroxyl Radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. In organic synthesis, hydroxyl radicals are most commonly generated by photolysis of 1-hydroxy-2(1''H'')-pyridinethione. Notation The unpaired electron of the hydroxyl radical is officially represented by a middle dot, •, beside the O. Biology Hydroxyl radicals can occasionally be produced as a byproduct of immune action. Macrophages and microglia most frequently generate this compound when expos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bonds
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. The strength of chemical bonds varies considerably; there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force and hydrogen bonding. Strong chemical bonding arises from the sharing or transfer of electrons between the participating atoms. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction const ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penetration Depth
Penetration depth is a measure of how deep light or any electromagnetic radiation can penetrate into a material. It is defined as the depth at which the intensity of the radiation inside the material falls to 1/e (about 37%) of its original value at (or more properly, just beneath) the surface. When electromagnetic radiation is incident on the surface of a material, it may be (partly) reflected from that surface and there will be a field containing energy transmitted into the material. This electromagnetic field interacts with the atoms and electrons inside the material. Depending on the nature of the material, the electromagnetic field might travel very far into the material, or may die out very quickly. For a given material, penetration depth will generally be a function of wavelength. Beer–Lambert law According to Beer–Lambert law, the intensity of an electromagnetic wave inside a material falls off exponentially from the surface as : I(z) = I_0 \, e^ If \delta_p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Event (particle Physics)
In particle physics, an event refers to the results just after a fundamental interaction takes place between subatomic particles, occurring in a very short time span, at a well-localized region of space. Because of the uncertainty principle, an event in particle physics does not have quite the same meaning as it does in the theory of relativity, in which an "event" is a point in spacetime which can be known exactly, i.e., a spacetime coordinate. Overview In a typical particle physics event, the incoming particles are scattered or destroyed, and up to hundreds of particles can be produced, although few are likely to be new particles not discovered before. In the old bubble chambers and cloud chambers, "events" could be seen by observing charged particle tracks emerging from the region of the event before they curl due to the magnetic field through the chamber acting on the particles. At modern particle accelerators, events are the result of the interactions which occur from a bea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterility (physiology)
Sterility is the physiological inability to effect sexual reproduction in a living thing, members of whose kind have been produced sexually. Sterility has a wide range of causes. It may be an inherited trait, as in the mule; or it may be acquired from the environment, for example through physical injury or disease, or by exposure to radiation. Sterility is the inability to produce a biological child, while infertility is the inability to conceive after a certain period. Sterility is rarely discussed in clinical literature and is often used synonymously with infertility. Infertility affects about 12-15% of couples globally. Still, the prevalence of sterility remains unknown. Sterility can be divided into three subtypes natural, clinical, and hardship. Natural sterility is the couple’s physiological inability to conceive a child naturally. Clinical sterility is natural sterility for which treatment of the patient will not result in conception. Hardship sterility is the inabi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)