|
Ring Strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated. Ring strain results from a combination of angle strain, conformational strain or Pitzer strain (torsional eclipsing interactions), and transannular strain, also known as van der Waals strain or Prelog strain. The simplest examples of angle strain are small cycloalkanes such as cyclopropane and cyclobutane. Ring strain energy can be attributed to the energy required for the distortion of bond and bond angles in order to close a ring. Ring strain energy is believed to be the cause of accelerated rates in altering ring reactions. Its interactions with traditional bond energi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Angles
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of a molecule, i.e. they can be understood as approximately local and hence transferable properties. Determination The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR, microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography, neutron diffraction and electron diffraction can give molecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Newman Projection
A Newman projection is a drawing that helps visualize the 3-dimensional structure of a molecule. This projection most commonly sights down a carbon-carbon bond, making it a very useful way to visualize the stereochemistry of alkanes. A Newman projection visualizes the Conformational isomerism, conformation of a chemical bond from front to back, with the front atom represented by the intersection of three lines (a dot) and the back atom as a circle. The front atom is called ''proximal'', while the back atom is called ''distal''. This type of representation clearly illustrates the specific dihedral angle between the proximal and distal atoms. This projection is named after American chemist Melvin Spencer Newman, who introduced it in 1952 as a partial replacement for Fischer projections, which are unable to represent conformations and thus conformers properly.Newman, MS. ''Record. Chem. Progr. (Kresge-Hooker Sci. Lib.) 1952,'' 13'', 111'' This diagram style is an alternative to a sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrophotometry
Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as spectrophotometers, that can measure the intensity of a light beam at different wavelengths. Although spectrophotometry is most commonly applied to ultraviolet, Visible spectrum, visible, and infrared radiation, modern spectrophotometers can interrogate wide swaths of the electromagnetic spectrum, including x-ray, ultraviolet, Visible spectrum, visible, infrared, or microwave wavelengths. Overview Spectrophotometry is a tool that hinges on the quantitative analysis of molecules depending on how much light is absorbed by colored compounds. Important features of spectrophotometers are spectral bandwidth (the range of colors it can transmit through the test sample), the percentage of sample transmission, the logarithmic range of sample absorptio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trans-Cyclooctene
''trans''-Cyclooctene is a cyclic compound, cyclic hydrocarbon with the formula [–(CH2)6CH=CH–], where the two C–C single bonds adjacent to the double bond are on opposite sides of the latter's plane. It is a colorless liquid with a disagreeable odor. Cyclooctene is notable as the smallest cycloalkene that is readily isolated as its ''trans''-isomer. The ''cis''-isomer is much more stable; the ring-strain energies being 16.7 and 7.4 kcal/mol, respectively. A planar arrangement of the ring carbons would be too strained, and therefore the stable conformational isomer, conformations of the ''trans'' form have a bent (non-planar) ring. Computations indicate that the most stable "crown" conformation has the carbon atoms alternately above and below the plane of the ring. A "half-chair" conformation, with about 6 kcal/mol higher energy, has carbons 2,3,5,6, and 8 on the same side of the plane of carbons 1,4, and 7. All conformations of ''trans''-cyclooctene are chirality, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norbornene Isomers Bredt Rule
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carries a double bond which induces significant ring strain and significant reactivity. Production Norbornene is made by a Diels–Alder reaction of cyclopentadiene and ethylene. Many substituted norbornenes can be prepared similarly. Related bicyclic compounds are norbornadiene, which has the same carbon skeleton but with two double bonds, and norbornane which is prepared by hydrogenation of norbornene. Reactions Norbornene undergoes an acid-catalyzed hydration reaction to form norborneol. This reaction was of great interest in the elucidation of the non-classical carbocation controversy. Norbornene is used in the Catellani reaction and in norbornene-mediated ''meta''-C−H activation. Certain substituted norbornenes undergo unusual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
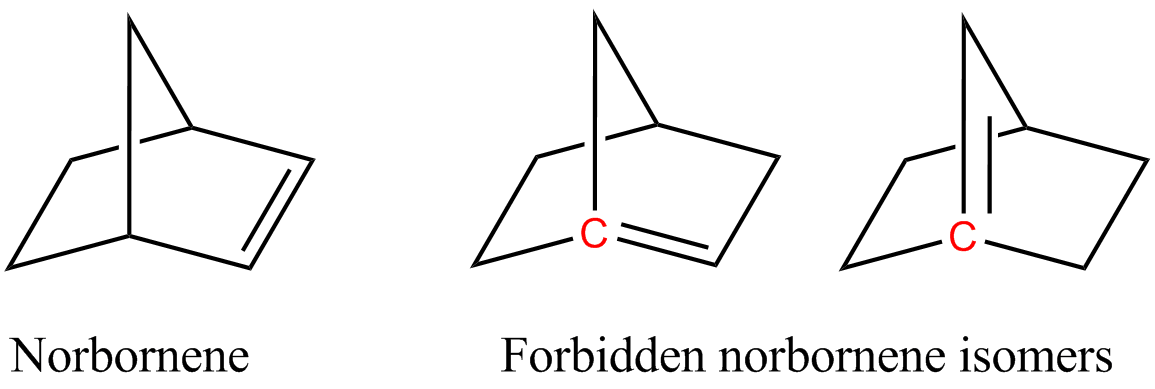
Bredt's Rule
In organic chemistry, an anti-Bredt molecule is a Bridged compound, bridged molecule with a double bond at the Bicyclic molecule, bridgehead. Bredt's rule is the empirical observation that such molecules only form in large ring systems. For example, two of the following norbornene isomers violate Bredt's rule, and are too unstable to prepare: The rule is named after Julius Bredt, who first discussed it in 1902 and codified it in 1924. There are a few instances where the anti-Bredt phenomenon is mentioned, but the isolation of these molecules is difficult, so they are typically trapped in situ. In pioneering studies, Wiseman, Keese, Wiberg, and others validated the intermediacy of anti-Bredt olefins beginning in the 1960s. Authors such as Mehta (2002) and Khan (2015) also obtained some possible support for the intermediacy of anti-Bredt olefins. In 2024, Neil Garg and his team demonstrated that the formation of anti-Bredt molecules is possible, even if only as short-lived intermed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula . It has a cage-like fused-ring structure ( truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a football. Each of its 60 carbon atoms is bonded to its three neighbors. Buckminsterfullerene is a black solid that dissolves in hydrocarbon solvents to produce a violet solution. The substance was discovered in 1985 and has received intense study, although few real world applications have been found. Molecules of buckminsterfullerene (or of fullerenes in general) are commonly nicknamed buckyballs. Occurrence Buckminsterfullerene is the most common naturally occurring fullerene. Small quantities of it can be found in soot. It also exists in space. Neutral has been observed in planetary nebulae and several types of star. The ionised form, , has been identified in the interstellar medium, where it is the cause of several absorption features known as diffuse interstellar bands in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkane
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the ring (chemistry), monocyclic Saturated and unsaturated compounds, saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring (possibly with side chains), and all of the carbon-carbon bonds are single bond, single. The larger cycloalkanes, with more than 20 carbon atoms are typically called ''cycloparaffins''. All cycloalkanes are isomers of alkenes. The cycloalkanes without side chains (also known as monocycloalkanes) are classified as small (cyclopropane and cyclobutane), common (cyclopentane, cyclohexane, and cycloheptane), medium (cyclooctane through cyclotridecane), and large (all the rest). Besides this standard definition by IUPAC, the International Union of Pure and Applied Chemistry (IUPAC), in some authors' usage the term ''cycloalkane'' includes also those saturated hydrocarbons th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylene Group
A methylene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ... atom, which is connected to the remainder of the molecule by two single bonds. The group may be represented as or , where the '>' denotes the two bonds. This stands in contrast to a situation where the carbon atom is bound to the rest of the molecule by a double bond, which is preferably called a methylidene group, represented . Formerly the methylene name was used for both isomers. The name “ methylene bridge“ can be used for the single-bonded isomer, to emphatically exclude methylidene. The distinction is often important, because the double bond is chemically different from two single bonds. The methylene group should be d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some Stress (mechanics), stress which raises its internal energy in comparison to a strain-free reference Chemical compound, compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional amount of internal energy which an unstrained molecule does not. This extra internal energy, or strain energy, can be likened to a compression (physics), compressed spring (device), spring.Anslyn and Dougherty, ''Modern Physical Organic Chemistry'', University Science Books, 2006, Much like a compressed spring must be held in place to prevent release of its potential energy, a molecule can be held in an energetically unfavorable conformation by the Chemical bond, bonds within that molecule. Without the bonds holding the conformation in place, the strain energy would be released. Summary Thermodynamics The Chemical equilibrium, equilibrium of two c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |