|
Rhodium Carbonyl Chloride
Rhodium carbonyl chloride is an organorhodium compound with the formula Rh2Cl2(CO)4. It is a red-brown volatile solid that is soluble in nonpolar organic solvents. It is a precursor to other rhodium carbonyl complexes, some of which are useful in homogeneous catalysis. Structure The molecule consists of two planar Rh(I) centers linked by two bridging chloride ligands and four CO ligands. X-ray crystallography shows that the two Rh(I) centers are square planar with the dihedral angle of 126.8° between the two RhCl2 planes. The metals are nonbonding. Synthesis and reactions First prepared by Walter Hieber, it is typically prepared by treating hydrated rhodium trichloride with flowing carbon monoxide, according to this idealized redox equation: :2 RhCl3(H2O)3 + 6 CO → Rh2Cl2(CO)4 + 2 COCl2 + 6 H2O.McCleverty, J. A.; Wilkinson, G. "Dichlorotetracarbonyldirhodium (rhodium carbonyl chloride)" Inorganic Syntheses 1966, volume 8, pp. 211-14. The complex reacts with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organorhodium Compound
Organorhodium chemistry is the chemistry of organometallic compounds containing a rhodium-carbon chemical bond, and the study of rhodium and rhodium compounds as catalysts in organic reactions. Stable organorhodium compounds and transient organorhodium intermediates are used as catalyst such as in olefin hydroformylation, olefin hydrogenation, olefin isomerization and the Monsanto process. Classification based on principal oxidation states Organometallic rhodium compounds share many characteristics with those of iridium, but less so with cobalt. Rhodium can exist in oxidation states of −3 to +5, but rhodium(I) and rhodium(III) are the more common. Rhodium(I) compounds (d8 configuration) usually occur with square planar or trigonal bipyramidal geometries, while rhodium (III) compounds (d6 configuration) typically have an octahedral geometry. Rhodium(0) Rhodium(0) complexes are binary carbonyls, the principal examples being tetrarhodium dodecacarbonyl, Rh4(CO)12, and hexadecacarbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is classified as a 1,3-diketone. It exists in equilibrium with a tautomer . The mixture is a colorless liquid. These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. Acetylacetone is a building block for the synthesis of many coordination complexes as well as heterocyclic compounds. Properties Tautomerism The Keto–enol tautomerism, keto and enol tautomers of acetylacetone coexist in solution. The enol form has C2v molecular symmetry, symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. In the gas phase, the equilibrium constant, ''K''keto→enol, is 11.7, favoring the enol form. The two tautomeric forms can be distinguished by NMR spectroscopy, IR spectroscopy and other methods. The equilibrium constant tends to be high in nonpolar solvents; when ''K''keto→enol is equal or greater than 1, the enol form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Complexes
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes, ketones and carboxylic acid), as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, such that carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides, chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimers (chemistry)
Dimers is a sports betting analytics platform that provides predictive tools, data-driven insights, news, and betting content for sports fans and bettors. Operating under the umbrella of Cipher Sports Technology Group, Dimers has offices in Melbourne and New York. History Dimers.com was launched on August 1, 2020, shortly after its parent company Cipher Sports Technology Group was formed through the merger of two Australian companies: iRival Media and Hypometer Technologies. iRival Media, founded in October 2019 by Adam Fiske and Nick Slade, focused on delivering content to the sports betting market.Hypometer Technologies, established in 2015 by Katie Prowd and Darryl Woodford, specialized in predictive analytics and machine learning for sports. Dimers’ data has been cited by publications such as ''Sports Illustrated,'' ''USA Today,'' and ''The Arizona Republic ''The Arizona Republic'' is an American daily newspaper published in Phoenix. Circulated throughout Arizo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogeneous Catalysis
In chemistry, homogeneous catalysis is catalysis where the catalyst is in same phase as reactants, principally by a soluble catalyst in a solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid and gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is an established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts. Examples Acid catalyst The proton is a pervasive homogeneous catalyst because water is the most common solvent. Water forms protons by the process of self-ionization of water. In an illustrative case, acids accelerate (catalyze) the hydrolysis of esters: :CH3CO2CH3 + H2O CH3CO2H + CH3OH At neutral pH, aqueous solutions of most esters do not hydrolyze at practical rates. Transition met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organorhodium Compounds
Organorhodium chemistry is the chemistry of organometallic compounds containing a rhodium-carbon chemical bond, and the study of rhodium and rhodium compounds as catalysts in organic reactions. Stable organorhodium compounds and transient organorhodium intermediates are used as catalyst such as in olefin hydroformylation, olefin hydrogenation, olefin isomerization and the Monsanto process. Classification based on principal oxidation states Organometallic rhodium compounds share many characteristics with those of iridium, but less so with cobalt. Rhodium can exist in oxidation states of −3 to +5, but rhodium(I) and rhodium(III) are the more common. Rhodium(I) compounds (d8 configuration) usually occur with square planar or trigonal bipyramidal geometries, while rhodium (III) compounds (d6 configuration) typically have an octahedral geometry. Rhodium(0) Rhodium(0) complexes are binary carbonyls, the principal examples being tetrarhodium dodecacarbonyl, Rh4(CO)12, and hexadecacarbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
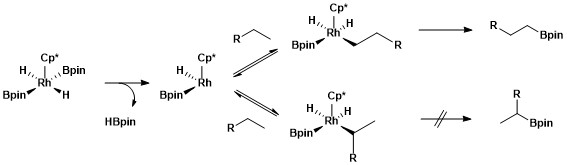
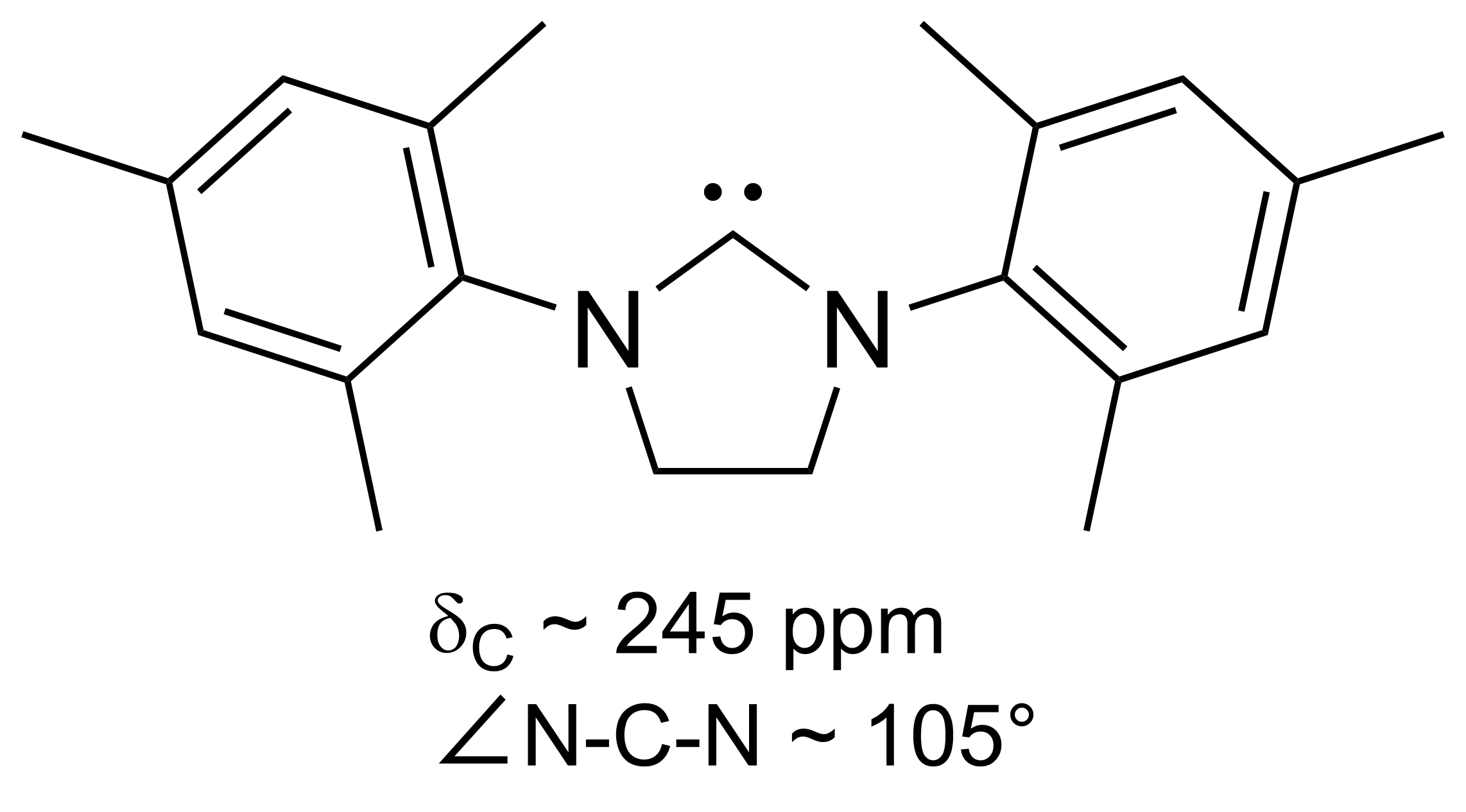
N-heterocyclic Carbene
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with octet rule, incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with such moieties. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), in which nitrogen atoms flank the formal carbene. Modern theoretical analysis suggests that the term "persistent carbene" is in fact a misnomer. Persistent carbenes do not in fact have a carbene electronic structure in their ground state, but instead an ylide stabilized by Aromaticity, aromatic resonance or steric shielding. Excitation to a carbene structure then accounts for the carbene-like dimerization that some persistent carbenes undergo over the course of days. Persistent carbenes in general, and Arduengo carbenes in particular, are popular ligands in organometallic chemistry. Histor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ECW Model
In chemistry, the ECW model is a semi-quantitative model that describes and predicts the strength of Lewis acid–Lewis base interactions. Many chemical reactions can be described as acid–base reactions, so models for such interactions are of potentially broad interest. The model initially assigned E and C parameters to each and every acid and base. The model was later expanded to ''the ECW model'' to cover reactions that have a constant energy term, ''W'', which describes processes that precede the acid–base reaction. This quantitative model is often discussed with the qualitative HSAB theory, which also seeks to rationalize the behavior of diverse acids and bases. History of the problem As early as 1938, G. N. Lewis pointed out that the relative strength of an acid or base depended upon the base or acid against which it was measured. No single rank order of acid or base strength can predict the energetics of the cross reaction. Consider the following pair of acid–base re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrothiophene
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. The molecule consists of a five-membered saturated ring with four methylene groups and a sulfur atom. It is the saturated analog of thiophene and is therefore the sulfur analog of THF. It is a volatile, colorless liquid with an intensely unpleasant odor. It is also known as thiophane, thiolane, or THT. Synthesis and reactions Tetrahydrothiophene is prepared by the reaction of tetrahydrofuran with hydrogen sulfide. This vapor-phase reaction is catalyzed by alumina and other heterogenous acid catalysts. This compound is a ligand in coordination chemistry, an example being the complex chloro(tetrahydrothiophene)gold(I). Oxidation of THT gives the sulfone sulfolane, which is of interest as a polar, odorless solvent: : Sulfolane is, however, more conventionally prepared from butadiene. Natural occurrence Both unsubstituted and substituted tetrahydrothiophenes are reported to occur in nature. For examp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicarbonyl(acetylacetonato)rhodium(I)
Dicarbonyl(acetylacetonato)rhodium(I) is an organorhodium compound with the formula Rh(O2C5H7)(CO)2. The compound consists of two CO ligands and an acetylacetonate. It is a dark green solid that dissolves in acetone and benzene, giving yellow solutions. The compound is used as a precursor to homogeneous catalysts. It is prepared by treating rhodium carbonyl chloride with sodium acetylacetonate in the presence of base: : CO)2RhClsub>2 + 2 NaO2C5H7 → 2 Rh(O2C5H7)(CO)2 + 2 NaCl The complex adopts square planar molecular geometry In chemistry, the square planar molecular geometry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the co .... The molecules stack with Rh---Rh distances of about 326 pm. As such, it is representative of a linear chain compound. References {{Acetylacetonate complexes Organorhodium compounds Homog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Complex
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes, ketones and carboxylic acid), as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, such that carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides, chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |